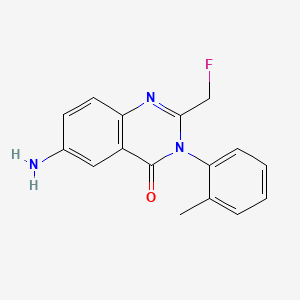
Afloqualone
| Molecular Formula: | C16H14FN3O |
|---|---|
| Molecular Weight: | 283.306 g/mol |
6-amino-2-(fluoromethyl)-3-(2-methylphenyl)quinazolin-4-one
HQ 495, C033541, QA-3735, UNII:CO4U2C8ORZ
Afloqualone; 56287-74-2; Arofuto; Aroft; Afloqualon; Afloqualone [INN:JAN]
Afloqualone (Arofuto) is a quinazolinone family GABAergic drug and is an analogue of methaqualone developed in the 1970s by a team at Tanabe Seiyaku.[1] It has sedative and muscle-relaxant effects resulting from its agonist activity at the β subtype of the GABAareceptor ,[2] and has had some clinical use, although it causes photosensitization as a side-effect that can cause skin problems such as dermatitis.[3]
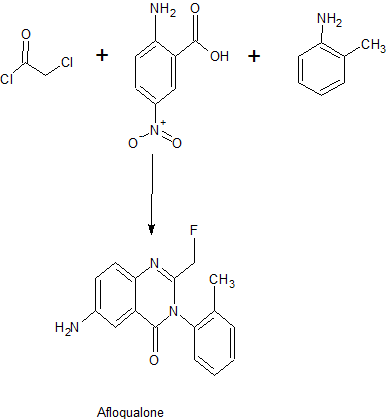
PATENT
CN 106496145
PATENT
CN 106496144
https://patents.google.com/patent/CN106496144A/en
Example 1:
[0027] A-fluoro-quinolin-one process, comprising the steps of:
[0028] A. To the hydrogenation apparatus 10g 6- nitro-2- (fluoromethyl) -3- (2-methylphenyl) -4- (3H) -1,3- phthalazinone , 150ml acid content of 0.1 ~ 0.4N n-butanol solution of acetic acid, lg palladium ruthenium bimetallic catalyst, hydrogen pressure 0.02 ~ 0.4MPa, reaction temperature of 25-50 ° C, after 1 hour, filtered to give the filtrate ;
[0029] B. washed catalyst with ethanol, at normal temperature, under reduced pressure to obtain a solution ⑴;
[0030] C. was added to the filtrate and the solution ⑴ water, 0.1N sodium hydroxide solution was added, the pH adjusted to 10.2 to 11.0, and stirred at 50-60 ° C 0.5 h, cooled to room temperature and filtered to give the crude fluoro-quinolin-one;
[0031] D.-fluoro-quinolin per gram of the recrystallization solvent was added 5 ~ 15ml crude ketone, wherein the recrystallization solvent is a volume ratio of 1: 1: 0.2 in a solution of isopropanol (m), a solution of acid butyl ester (II ) and water mixture; crystallized at room temperature, filtered to give a fluorine methaqualone.
[0032] Example 2:
[0033] A-fluoro-quinolin-one process, comprising the steps of:
[0034] A. hydrogenation apparatus added to 20g 6- nitro _2_ (fluoromethyl) -3- (2_-methylphenyl) -4- (3-1,3-phthalazinone buckle, acid content of the acid-containing 240ml 0.1 ~ 0.4N ethanol solution of hydrochloric acid, lg palladium ruthenium bimetallic catalyst, hydrogen pressure 0.02 ~ 0.4MPa, reaction temperature of 25-50 ° C. after 0.5 hours the reaction was filtered to obtain filtrate;
[0035] B. washed catalyst with ethanol, at normal temperature, under reduced pressure to obtain a solution ⑴;
[0036] C. was added to the filtrate and the solution ⑴ water, 0.1N sodium hydroxide solution was added, the pH adjusted to 10.2 to 11.0, and stirred at 50-60 ° C 1 hour, cooled to room temperature and filtered to give the crude fluoro-quinolin-one;
[0037] D.-fluoro-quinolin added per gram of crude ketone was recrystallized from 5 ~ 15ml of the solvent, wherein the recrystallization solvent is a volume ratio of 1: o.2: methanol solution of i (m), an ethyl acetate solution (II ) and water mixture; crystallized at room temperature, filtered to give a fluorine methaqualone.
[0038] Example 3:
[0039] – quinolin-fluoro-one kind of process, comprising the steps of:
[0040] A. Add 5g 6- nitro apparatus _2_ hydride (fluoromethyl) -3- (2-methylphenyl) -4- (3-1,3-Perot phthalazinone, 80ml methanol containing an acid in an amount of 0.1 ~ 0.4N solution of sulfuric acid, lg palladium ruthenium bimetallic catalyst, hydrogen pressure 0.02 ~ 0.4MPa, reaction temperature of 25-50 ° C. after 1.5 hours the reaction was filtered to obtain filtrate;
[0041] B. the catalyst was washed with ethanol, normal temperature under reduced pressure to obtain a solution (the I);
[0042] C. was added to the filtrate and the solution (I) water, 0.1N sodium hydroxide solution was added, the pH adjusted to 10.2 to 11.0, and stirred at 50-60 ° C 1 hour, cooled to room temperature and filtered to give fluoro-quinolin-one Crude;
[0043] D. methaqualone fluorine per gram of crude product were added 5 ~ 15ml recrystallization solvent, wherein the recrystallization solvent is a volume ratio of 1: 0.2: 0.2 ethanol solution (m), carboxylic acid butyl ester (II) and water mixture; crystallization at room temperature, and filtered to give fluoro-quinolin-one.
PAPER
6-Amino-2-(fluoromethyl)-3-(2-methylphenyl)quinazolin-4(3H)-one
Acta Crystallographica, Section E: Structure Reports Online (2007), 63, (7), o3109
http://scripts.iucr.org/cgi-bin/paper?S1600536807026670
PAPER
Synthesis of the metabolites of afloqualone and related compounds
Chemical & pharmaceutical bulletin (1983), 31, (4), 1158-65.
Seven main metabolites (3-9) of afloqualone (1, 6-amino-2-fluoromethyl-3-(o-tolyl)-4 (3H)-quinazolinone and related 4 (3H)-quinazolinone derivatives were synthesized. The metabolites 4 and 5 containing a sulfur atom were prepared by the reaction of 6-acetamido-2-chloromethyl-3-(o-tolyl)-4 (3H)-quinazolinone (11) with NaSCH3 followed by oxidation with H2O2. Reaction of 11 and N-acetyl-L-cysteine gave the mercapturic acid-conjugated metabolite 6. Condensation of 2-fluoroacetamido-5-nitrobenzoic acid (19) and 2-amino-benzyl alcohol (20) with dicyclohexylcarbodiimide (DCC) in the presence of 1-hydroxy-benzotriazole afforded 2-fluoromethyl-3-(o-hydroxymethylphenyl)-6-nitro-4 (3H)-quinazolinone (21), which was converted to the metabolites 7 and 8. Treatment of the 2-bromomethyl-4 (3H)-quinazolinone (24) with AgBF4-H2O in dimethylsulfoxide (DMSO) gave the 2-hydroxymethyl metabolite 9. None of the main metabolites (2-9) showed significant central nervous system depressant activity
https://www.jstage.jst.go.jp/article/cpb1958/31/4/31_4_1158/_article
References
- Jump up^ US Patent 3966731 – 2-Fluoromethyl-3-o-tolyl-6-amino-4(3H)-quinazolinone
- Jump up^ Ochiai T, Ishida R. Pharmacological studies on 6-amino- 2-fluoromethyl- 3-(O-tolyl)- 4(3H)- quinazolinone (afloqualone), a new centrally acting muscle relaxant. (II) Effects on the spinal reflex potential and the rigidity. Japanese Journal of Pharmacology. 1982 Jun;32(3):427-38.
- Jump up^ Ishikawa T, Kamide R, Niimura M. Photoleukomelanodermatitis (Kobori) induced by afloqualone. Journal of Dermatology. 1994 Jun;21(6):430-3.
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H14FN3O |
| Molar mass | 283.3 |
| 3D model (JSmol) | |
//////////////Afloqualone, HQ 495, アフロクアロン , C033541, QA-3735, UNII:CO4U2C8ORZ, 4831
CC1=CC=CC=C1N2C(=NC3=C(C2=O)C=C(C=C3)N)CF
Afloqualone
- ATC:M03A
- MW:283.31 g/mol
- CAS-RN:56287-74-2
- LD50:397 mg/kg (M, p.o.);
249 mg/kg (R, p.o.)
Derivatives
hydrochloride
- Formula:C16H14FN3O • xHCl
- MW:unspecified
- CAS-RN:56287-75-3
Substance Classes
Synthesis Path
Substances Referenced in Synthesis Path
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 108-24-7 | C4H6O3 | acetic anhydride | Acetic acid, anhydride |
| 69123-71-3 | C7H5ClN2O3 | 2-amino-5-nitrobenzoyl chloride | Benzoyl chloride, 2-amino-5-nitro- |
| 23076-31-5 | C14H13N3O3 | N-(2-amino-5-nitrobenzoyl)-o-toluidine | Benzamide, 2-amino-N-(2-methylphenyl)-5-nitro- |
| 56287-72-0 | C16H14FN3O4 | 2-[(fluoroacetyl)amino]-N-(2-methylphenyl)-5-nitrobenzamide | Benzamide, 2-[(fluoroacetyl)amino]-N-(2-methylphenyl)-5-nitro- |
| 359-06-8 | C2H2ClFO | fluoroacetyl chloride | Acetyl chloride, fluoro- |
| 56287-73-1 | C16H12FN3O3 | 2-(fluoromethyl)-3-(2-methylphenyl)-6-nitro-4(3H)-quinazolinone | 4(3H)-Quinazolinone, 2-(fluoromethyl)-3-(2-methylphenyl)-6-nitro- |
| 616-79-5 | C7H6N2O4 | 5-nitroanthranilic acid | Benzoic acid, 2-amino-5-nitro- |
| 95-53-4 | C7H9N | o-toluidine | Benzenamine, 2-methyl- |
Trade Names
| Country | Trade Name | Vendor | Annotation |
|---|---|---|---|
| J | Aflomus | Hishiyama | |
| Airomate | SawaiNippon Chemiphar | ||
| Arofuto | Tanabe |
Formulations
- tabl. 20 mg
References
-
- Tani, J. et al.: J. Med. Chem. (JMCMAR) 22, 95 (1979).
- DOS 2 449 113 (Tanabe; appl. 15.10.1974; J-prior. 15.10.1973).
- US 3 966 731 (Tanabe; 29.6.1976; J-prior. 15.10.1973)