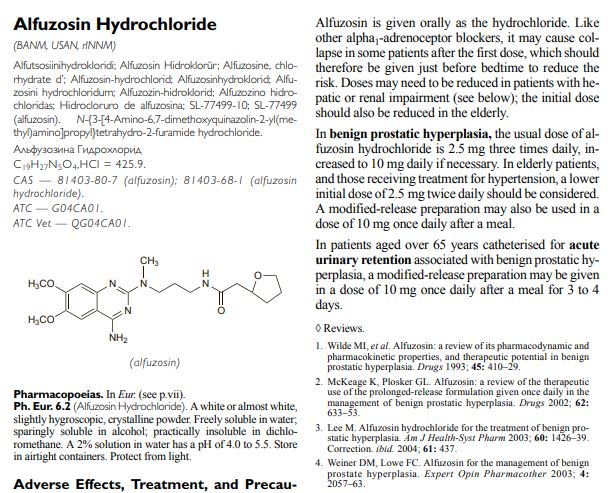
Alfuzosin
- Molecular FormulaC19H27N5O4
- Average mass389.449 Da
CAS
| CAS: | 81403-68-1 HCL SALT |

Alfuzosin hydrochloride (CAS 81403-68-1)
Alfuzosin (INN, provided as the hydrochloride salt) is a pharmaceutical drug of the α1 blocker class. As an antagonist of the α1adrenergic receptor, it works by relaxing the muscles in the prostate and bladder neck, making it easier to urinate. It is thus used to treat benign prostatic hyperplasia (BPH).[1]
Alfuzosin is marketed in the United States by Sanofi Aventis under the brand name Uroxatral and elsewhere under the tradenames Xat, Xatral, Prostetrol and Alfural. Alfuzosin was approved by the U.S. FDA for treatment of BPH in June 2003.
Side effects
The most common side effects are dizziness (due to postural hypotension), upper respiratory tract infection, headache, fatigue, and abdominal disturbances. Side effects include stomach pain, heartburn, and congested nose.[2] Adverse effects of alfuzosin are similar to that of tamsulosin with the exception of retrograde ejaculation.[3]
Contraindications
Alfuzosin should be used with caution in patients with severe renal insufficiency, and should not be prescribed to patients with a known history of QT prolongation who are taking medications known to prolong the QT interval.
Chemistry
Alfuzosin contains a stereocenter and is therefore chiral. There are two enantiomeric forms, (R)-alfuzosin and (S)-alfuzosin. The drug is used as a racemate, (RS)-alfuzosin, a 1: 1 mixture of the (R)- and (S)-forms.[4]
| Enantiomers of alfuzosin | |
|---|---|
 CAS number: 123739-69-5 |
 CAS number.: 123739-70-8 |
Alfuzosin
- ATC:G04CA01
- MW:389.46 g/mol
- CAS-RN:81403-80-7
Derivatives
monohydrochloride
- Formula:C19H27N5O4 • HCl
- MW:425.92 g/mol
- CAS-RN:81403-68-1
Substance Classes
Synthesis Path
Substances Referenced in Synthesis Path
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 23680-84-4 | C10H10ClN3O2 | 4-amino-2-chloro-6,7-dimethoxyquinazoline | 4-Quinazolinamine, 2-chloro-6,7-dimethoxy- |
| 5004-88-6 | C9H12N2O3 | 2-amino-4,5-dimethoxybenzamide | Benzamide, 2-amino-4,5-dimethoxy- |
| 541-41-3 | C3H5ClO2 | chloroformic acid ethyl ester | Carbonochloridic acid, ethyl ester |
| 72104-44-0 | C9H14N2O2 | 2-cyano-N-methyl-N-tetrahydrofuroylethylamine | 2-Furancarboxamide, N-(2-cyanoethyl)tetrahydro-N-methyl- |
| 27631-29-4 | C10H8Cl2N2O2 | 2,4-dichloro-6,7-dimethoxyquinazoline | Quinazoline, 2,4-dichloro-6,7-dimethoxy- |
| 28888-44-0 | C10H10N2O4 | 2,4-dihydroxy-6,7-dimethoxyquinazoline | 2,4(1H,3H)-Quinazolinedione, 6,7-dimethoxy- |
| 20357-25-9 | C9H9NO5 | 4,5-dimethoxy-2-nitrobenzaldehyde | Benzaldehyde, 4,5-dimethoxy-2-nitro- |
| 4959-60-8 | C9H10N2O5 | 4,5-dimethoxy-2-nitrobenzamide | Benzamide, 4,5-dimethoxy-2-nitro- |
| 28888-44-0 | C10H10N2O4 | 6,7-dimethoxyquinazoline-2,4-dione | 2,4(1H,3H)-Quinazolinedione, 6,7-dimethoxy- |
| 541-41-3 | C3H5ClO2 | ethyl chloroformate | Carbonochloridic acid, ethyl ester |
| 693-05-0 | C4H8N2 | 3-(methylamino)propanenitrile | Propanenitrile, 3-(methylamino)- |
| 81403-67-0 | C9H18N2O2 | N1-methyl-N2-tetrahydrofuroyltrimethylenediamine | 2-Furancarboxamide, tetrahydro-N-[3-(methylamino)propyl]- |
| 16874-33-2 | C5H8O3 | (±)-tetrahydrofuran-2-carboxylic acid | 2-Furancarboxylic acid, tetrahydro- |
| 167391-50-6 | C8H12O5 | tetrahydro-2-furancarboxylic acid anhydride with ethyl hydrogen carbonate | 2-Furancarboxylic acid, tetrahydro-, anhydride with ethyl hydrogen carbonate |
| 57-13-6 | CH4N2O | urea | Urea |
| 120-14-9 | C9H10O3 | veratraldehyde | Benzaldehyde, 3,4-dimethoxy- |
Trade Names
| Country | Trade Name | Vendor | Annotation |
|---|---|---|---|
| D | Alfunar | Apogepha | |
| Alfusin | TAD Pharma | ||
| Urion | Sanofi-Aventis | ||
| Uroxatral | Sanofi-Aventis | ||
| F | Urion | Zambon | |
| Xatral | Sanofi-Aventis | ||
| GB | Xatral | Sanofi-Aventis | |
| I | Mittoval | Sanofi-Aventis | |
| Xatral | Sanofi-Aventis |
Formulations
- film tabl. 2.5 mg; retard tabl. 10 mg (hydrochloride)
References
-
- Manoury, P.M. et al.: J. Med. Chem. (JMCMAR) 29, 19 (1986).
- US 4 315 007 (Synthelabo; 9.2.1982; F-prior. 6.2.1978, 29.12.1978).
- DE 2 904 445 (Synthelabo; appl. 16.8.1979; F-prior. 6.2.1978, 29.12.1978).
-
synthesis of 6,7-dimethoxyquinazoline-2,4-dione:
- Althuis, T.H.; Hess, H.J.: J. Med. Chem. (JMCMAR) 20, 146 (1977).
SYN
Mathias Scheer, “Alfuzosin tablets and synthesis.” U.S. Patent US20060062845, issued March 23, 2006.
Syn, DOI: 10.1021/jm00151a003 NB: (WO2009001369)

FTIR spectrum of alfuzosin hydrochloride
CLIP
(±)-N-[3-[(4-Amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furamide monohydrochloride
USP Alfuzosin Hydrochloride RS.
USP Alfuzosin System Suitability Mixture RS.
Identification—
pH ![]() 791
791![]() : between 4.0 and 5.5
: between 4.0 and 5.5
Optical rotation ![]() 781
781![]() :
: ![]() 0.10
0.10![]() to +0.10
to +0.10![]()
Related compounds—
Chromatographic system (see Chromatography ![]() 621
621![]() )— The liquid chromatograph is equipped with a detector set at 254 nm and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the peak-to-valley ratio is at least 5. [NOTE—The peak-to-valley ratio is determined as the ratio of the height above the baseline of the impurity A peak to the height above the baseline of the lowest point of the curve separating this impurity peak from the peak due to alfuzosin.]
)— The liquid chromatograph is equipped with a detector set at 254 nm and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the peak-to-valley ratio is at least 5. [NOTE—The peak-to-valley ratio is determined as the ratio of the height above the baseline of the impurity A peak to the height above the baseline of the lowest point of the curve separating this impurity peak from the peak due to alfuzosin.]
Procedure— Separately inject equal volumes (about 10 µL) of the Reference solution and the Test solution, record the chromatograms, and measure the peak responses. Calculate the percentage of each impurity in the portion of Alfuzosin Hydrochloride taken by the formula:
in which 100 is the percentage conversion factor; rU is the peak response for any impurity obtained from the Test solution; 1000 is the dilution factor; and rS is the peak response for alfuzosin obtained from the Reference solution: the limits are as shown in the accompanying table. Disregard any peak with an area less than 0.05%.
| Compound | Relative Retention Time |
Limit (%) |
| Alfuzosin | 1.0 | — |
| Impurity A1 | 1.2 | * |
| Impurity D2 | 0.5 | 0.20 |
| Any individual unspecified impurity | — | 0.10 |
| Total impurities | — | 0.30 |
|
1 N-[3-[(4-Amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino]propyl]furan-2-carboxamide.
2 N-(4-Amino-6,7-dimethoxyquinazolin-2-yl)-N-methylpropane-1,3-diamine.
* Impurity A, a component of USP Alfuzosin System Suitability Mixture RS, is not a specified impurity.
|
||
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D. Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
Pharmacopeial Forum: Volume No. 34(1) Page 69
Chromatographic Column—
References
- Jump up^ Lepor, Herbert (2016). “Alpha-blockers for the Treatment of Benign Prostatic Hyperplasia”. Urologic Clinics of North America. 43 (3): 311–23. doi:10.1016/j.ucl.2016.04.009. PMC 2213889
 . PMID 27476124.
. PMID 27476124. - Jump up^ “Alfuzosin”. MedlinePlus. United States National Library of Medicine. April 15, 2016.
- Jump up^ Hills, Robert K; Liu, Chenli; Zeng, Guohua; Kang, Ran; Wu, Wenqi; Li, Jiasheng; Chen, Kang; Wan, Show P. (2015). “Efficacy and Safety of Alfuzosin as Medical Expulsive Therapy for Ureteral Stones: A Systematic Review and Meta-Analysis”. PLOS ONE. 10 (8): e0134589. doi:10.1371/journal.pone.0134589. ISSN 1932-6203. PMC 4526635
 . PMID 26244843.
. PMID 26244843. This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license. - Jump up^ Rote Liste Service GmbH (Hrsg.): Rote Liste 2017 – Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte). Rote Liste Service GmbH, Frankfurt/Main, 2017, Aufl. 57, S. 159, ISBN 978-3-946057-10-9.
External links
- Uroxatral (alfuzosin HCl) Extended-Release Tablets Prescribing Information
- Alfuzosin (General information from the NIH)
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ælˈfjuːzoʊsɪn/ al-FEW-zoh-sin |
| Trade names | Uroxatral, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a64002 |
| Pregnancy category |
|
| Routes of administration |
By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 49% |
| Protein binding | 82–90% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 10 hours |
| Excretion | Feces (69%) and Urine (24%) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.108.671 |
| Chemical and physical data | |
| Formula | C19H27N5O4 |
| Molar mass | 389.46 g·mol−1 |
| 3D model (JSmol) | |
/////////////////塩酸アルフゾシン, Uroxatral, alfuzosin
COC1=C(OC)C=C2C(N)=NC(=NC2=C1)N(C)CCCNC(=O)C1CCCO1
















