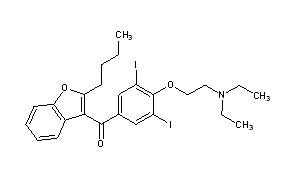

Amiodarone
CAS : 1951-25-3
(2-Butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone
2-butyl-3-benzofuranyl-4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone; 2-butyl-3-[3,5-diiodo-4-(b-diethylaminoethoxy)benzoyl]benzofuran
Molecular Formula: C25H29I2NO3
Molecular Weight: 645.31
Percent Composition: C 46.53%, H 4.53%, I 39.33%, N 2.17%, O 7.44%
In December 1985, amiodarone was approved by the FDA for the treatment of arrhythmias.[6] This makes amiodarone one of the few drugs approved by the FDA without rigorous randomized clinical trials.
Amiodarone is an antiarrhythmic agent used for various types of cardiac dysrhythmias, both ventricular and atrial. It was discovered in 1961. Despite relatively common side-effects, it is used in arrhythmias that are otherwise difficult to treat with medication.

A more recent synthesis of amiodarone reports the cyclisation of α-phenoxyhexanal 389 under acidic conditions to yield the substituted benzofuran 390 (Scheme 76). A Friedel–Crafts acylation next introduces the aryl ring at the 3-position. Demethylation, iodination and a final alkylation with a diethylaminoethane fragment yields amiodarone [115-117].
- 115 Witczak, M.; Kwiecień, H. Synth. Commun. 2005, 35, 2223–2230. doi:10.1080/00397910500182747
Return to citation in text: [1]
- Wang, Z. J. Synthetic Process for 2-Butyl-3-(hydroxy-3,5-diiodobenzoyl)-benzofuran. Chin. Patent 1,858,042, Nov 8, 2006……….116
Return to citation in text: [1]
- Ha, H. R.; Stieger, B.; Grassi, G.; Altorfer, H. R.; Follath, F. Eur. J. Clin. Pharmacol. 2000, 55, 807–814.doi:10.1007/s002280050701….117
Scheme 76: Synthesis of amiodarone……….http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-7-57#S76
Literature References:
Benzofuran derivative with multiple electrophysiological effects. Prepn: FR 1339389; R. Tondeur, F. Binon,US 3248401 (1963, 1966 to Soc. Belge l’Azote Prod. Chim. Marly).
Physicochemical properties: M. Bonati et al., J. Pharm. Sci. 73,829 (1984).
HPLC determn in plasma: M. De Smet, D. L. Massart, J. Pharm. Biomed. Anal. 6, 277 (1988).
Comprehensive description: T. A. Plomp, Anal. Profiles Drug Subs. 20, 1-120 (1991).
Review of pharmacology, clinical efficacy and safety: M. Chow, Ann. Pharmacother. 30, 637-643 (1996); B. N. Singh, Clin. Cardiol. 20, 608-618 (1997).
Clinical trial in cardiac resuscitation: P. J. Kudenchuk et al., N. Engl. J. Med. 341, 871 (1999); to prevent atrial fibrillation: D. Roy et al., ibid. 342, 913 (2000).
Derivative Type: Hydrochloride
CAS Registry Number: 19774-82-4
Manufacturers’ Codes: L-3428
Trademarks: Amiodar (Sanofi Winthrop); Ancaron (Taisho); Cordarex (Sanofi Winthrop); Cordarone (Wyeth); Ortacrone (Sanofi Winthrop); Pacerone (Upsher-Smith); Tachydaron (AWD); Trangorex (Sanofi Winthrop)
Molecular Formula: C25H29I2NO3.HCl
Molecular Weight: 681.77
Percent Composition: C 44.04%, H 4.44%, I 37.23%, N 2.05%, O 7.04%, Cl 5.20%
Properties: Crystalline powder, mp 156°. Also reported as crystals from acetone, mp 159 ±2° (Bonati). Soly at 25° (g/100ml): chloroform 44.51; methylene chloride 19.20; methanol 9.98; ethanol 1.28; benzene 0.65; tetrahydrofuran 0.60; acetonitrile 0.32; 1-octanol 0.30; ether 0.17; 1-propanol 0.13; water 0.07; hexane 0.03 petroleum ether 0.001. Sparingly sol in isopropanol; slightly sol in acetone, dioxane, and carbon tetrachloride. pH (5% soln) 3.4-3.9. pKa (25°C) 6.56 ±0.06. uv max (methanol): 208, 242 nm (E1%1cm 662 ±8, 623 ±10).
Melting point: mp 156°; mp 159 ±2° (Bonati)
pKa: pKa (25°C) 6.56 ±0.06
Absorption maximum: uv max (methanol): 208, 242 nm (E1%1cm 662 ±8, 623 ±10)
Therap-Cat: Antiarrhythmic (class III).
Keywords: Antiarrhythmic.

AMIODARONE
 |
|
|
Title: Amiodarone
CAS Registry Number: 1951-25-3
CAS Name: (2-Butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone
Additional Names: 2-butyl-3-benzofuranyl-4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone; 2-butyl-3-[3,5-diiodo-4-(b-diethylaminoethoxy)benzoyl]benzofuran
Molecular Formula: C25H29I2NO3
Molecular Weight: 645.31
Percent Composition: C 46.53%, H 4.53%, I 39.33%, N 2.17%, O 7.44%
Literature References: Benzofuran derivative with multiple electrophysiological effects. Prepn: FR 1339389; R. Tondeur, F. Binon, US 3248401 (1963, 1966 to Soc. Belge l’Azote Prod. Chim. Marly). Physicochemical properties: M. Bonati et al., J. Pharm. Sci. 73, 829 (1984). HPLC determn in plasma: M. De Smet, D. L. Massart, J. Pharm. Biomed. Anal. 6, 277 (1988). Comprehensive description: T. A. Plomp, Anal. Profiles Drug Subs. 20, 1-120 (1991). Review of pharmacology, clinical efficacy and safety: M. Chow, Ann. Pharmacother. 30, 637-643 (1996); B. N. Singh, Clin. Cardiol. 20, 608-618 (1997). Clinical trial in cardiac resuscitation: P. J. Kudenchuk et al., N. Engl. J. Med. 341, 871 (1999); to prevent atrial fibrillation: D. Roy et al., ibid. 342, 913 (2000).
Derivative Type: Hydrochloride
CAS Registry Number: 19774-82-4
Manufacturers’ Codes: L-3428
Trademarks: Amiodar (Sanofi Winthrop); Ancaron (Taisho); Cordarex (Sanofi Winthrop); Cordarone (Wyeth); Ortacrone (Sanofi Winthrop); Pacerone (Upsher-Smith); Tachydaron (AWD); Trangorex (Sanofi Winthrop)
Molecular Formula: C25H29I2NO3.HCl
Molecular Weight: 681.77
Percent Composition: C 44.04%, H 4.44%, I 37.23%, N 2.05%, O 7.04%, Cl 5.20%
Properties: Crystalline powder, mp 156°. Also reported as crystals from acetone, mp 159 ±2° (Bonati). Soly at 25° (g/100ml): chloroform 44.51; methylene chloride 19.20; methanol 9.98; ethanol 1.28; benzene 0.65; tetrahydrofuran 0.60; acetonitrile 0.32; 1-octanol 0.30; ether 0.17; 1-propanol 0.13; water 0.07; hexane 0.03 petroleum ether 0.001. Sparingly sol in isopropanol; slightly sol in acetone, dioxane, and carbon tetrachloride. pH (5% soln) 3.4-3.9. pKa (25°C) 6.56 ±0.06. uv max (methanol): 208, 242 nm (E1%1cm 662 ±8, 623 ±10).
Melting point: mp 156°; mp 159 ±2° (Bonati)
pKa: pKa (25°C) 6.56 ±0.06
Absorption maximum: uv max (methanol): 208, 242 nm (E1%1cm 662 ±8, 623 ±10)
Therap-Cat: Antiarrhythmic (class III).
Keywords: Antiarrhythmic.
|
Amiodarone
Use:antiarrhythmic
Chemical name:(2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone
Formula:C25H29I2NO3
- MW:645.32 g/mol
- CAS-RN:1951-25-3
- InChI Key:IYIKLHRQXLHMJQ-UHFFFAOYSA-N
- InChI:InChI=1S/C25H29I2NO3/c1-4-7-11-22-23(18-10-8-9-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-13-28(5-2)6-3/h8-10,12,15-16H,4-7,11,13-14H2,1-3H3
- EINECS:217-772-1
- LD50:178 mg/kg (M, i.v.); >4 g/kg (M, p.o.)
Derivatives
hydrochloride
- Formula:C25H29I2NO3 • HCl
- MW:681.78 g/mol
- CAS-RN:19774-82-4
Trade Names
| Country |
Trade Name |
Vendor |
Annotation |
| D |
Cordarex |
Sanofi-Aventis |
|
| Cornaron |
TAD Pharma |
|
| F |
Corbionax Ge |
Winthrop/Sanofi-Aventis |
|
| Cordarone |
Sanofi-Aventis |
|
| GB |
Cordaron |
Sanofi-Aventis |
|
| I |
Amiodar |
Sigma-Tau |
|
| Cordarone |
Sanofi-Aventis |
|
| J |
Ancaron |
Sanofi-Aventis |
|
| USA |
Cordarone |
Wyeth-Ayerst |
as hydrochloride |
Formulations
- inj. sol. 150 mg/3ml; tabl. 100 mg, 200 mg
References
-
- FR 1 339 389 (Labaz; appl. 22.11.1962).
- US 3 248 401 (Labaz; 26.4.1966; D-prior. 24.11.1961).
-
2-butylbenzofuran:
- Buu-Hoï, N.P. et al.: J. Chem. Soc. (JCSOA9) 1964, 173.
PATENT
CN109053652-PREPARATION METHOD OF AMIODARONE HYDROCHLORIDE INTERMITTENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN235615504&_cid=P11-KL0AU0-06410-1
| Amiodarone hydrochloride is currently the most widely used antiarrhythmic drug. In addition, amiodarone hydrochloride has become the first choice for long-term medication for patients with arrhythmia due to its stable curative effect and minor side effects. In today’s society, people’s work rhythm is constantly increasing, the pressure they face is increasing, the number of patients with cardiovascular diseases is increasing, the demand for anti-arrhythmic drugs is greatly increasing, and the market share of amiodarone hydrochloride is bound to continue to increase. |
| 2-Butyl-3-(4-hydroxybenzoyl)benzofuran is an important intermediate of amiodarone hydrochloride. Patent CN104262304 uses compounds 1 and 2 as starting materials, and the resulting compound 3 is reacted with sodium methoxide as a base and toluene as a solvent to form compound 5; compound 5 is reacted with compound 7 under Lewis acid conditions to form compound 8; compound 8 Hydrolyzed under Lewis acid conditions, the reaction produces 2-butyl-3-(4-hydroxybenzoyl)benzofuran 9. |
| This synthetic route has the following shortcomings: Compound 3 can be directly obtained without hydrolysis under the condition of sodium methoxide as base and toluene as solvent. However, the difficulty of demethylation is much greater than that of decarboxylation, resulting in the formation of compound 5. The conversion rate of the cyclization reaction is low, the by-products are many, and the separation is difficult; resulting in low total yield and increased cost. |
| The synthetic route reported in patent CN107382925, except that the route for preparing compound 5 from compound 3 is different, other reaction routes are the same, but the reaction conditions are different. In this reaction route, the aldehyde group of compound 3 is first protected with trimethyl orthoformate to produce compound 0, and then compound 0 is hydrolyzed to produce compound 4. Compound 4 is cyclized under the catalysis of p-toluenesulfonyl chloride to obtain compound 5. |
 |
| This route has the following disadvantages: when compound 3 is prepared to compound 5, trimethyl orthoformate is added to protect the aldehyde group, then the ester group is first hydrolyzed to form an acid, and then the protective group of the aldehyde group is removed; although side reactions of the aldehyde group can be reduced, but The reaction steps are added, and the trimethyl orthoformate is highly flammable and has potential safety hazards. The yield of compound 4 to compound 5 catalyzed by p-toluenesulfonyl chloride is low, and the cost is also increased. |

| Add 15.00kg (122.8mol, 1eq) compound 1 and 30.82kg (147.4mol, 1.2eq) compound 2 to 120.00kg (8w/w) ethyl acetate, add 24.00kg (73.7mol, 0.6eq) cesium carbonate, 1.00 kg (2.5 mol, 0.02 eq) methyl trioctyl ammonium chloride, stirred and heated to 75-85°C, reacted for 1 to 2 hours. After the reaction, the filtrate was filtered with suction, and the filtrate was washed with 60.00kg (4w/w) purified water, and the organic phase was concentrated to dryness under reduced pressure at 40°C to obtain 30.21kg (120.7mol) of compound 3 with a yield of about 98.3%. |
| 30.00kg (120.0mol, 1eq) of compound 3 was added to 14.40kg (360.0mol, 3eq) of 150.00kg (5w/w) aqueous solution of sodium hydroxide, and stirred at 20-30°C. After the reaction, add 1N dilute hydrochloric acid to adjust the pH to 4, add 30.00kg (1w/w) ethyl acetate for extraction, add 30.00kg (1w/w) for the organic phase and wash once with purified water, and add 30.00kg (1w/w) for the organic phase Wash with saturated sodium chloride aqueous solution, add 1.50kg (0.05w/w) anhydrous sodium sulfate to dry. The organic phase was concentrated to dryness under reduced pressure at 40° C. to obtain 27.30 kg (115.6 mol) of compound 4 with a yield of about 96.4%. |
| Add 27.00kg (114.3mol, 1eq) of compound 4 to 216.00kg (8.0w/w) of acetic anhydride, add 75.60kg (2.8w/w) of sodium acetate, raise the temperature to 90~100℃ for reaction, and react for 1~2 hours . After the reaction, the reaction solution was transferred to 270.00kg (10w/w) purified water, stirred for 2 hours, and then extracted with 81.00kg (3w/w) ethyl acetate. The organic phase was washed twice with 27.00kg (1.0w/w) purified water, and the organic phase was washed with 27.00kg (1.0w/w) saturated aqueous sodium chloride solution, and dried with 2.70kg (0.1w/w) anhydrous sodium sulfate. The organic phase was concentrated to dryness under reduced pressure at 40°C. 14.26kg (81.8mol) of compound 5 was obtained, and the yield was about 71.6%. 1 HNMR(400MHz,d DMSO )δ:0.92~0.96(t,3H,-CH 3 ),1.37~1.42(m,2H,-CH 2 CH 3 ),1.68~1.72(m,2H,-CH 2 CH 2 CH 3 ),2.77~2.81(t,2H,Ar-CH 2 -CH 2 ), 6.59 (s, 1H, -ArH), 7.20 ~ 7.24 (m, 2H, ArH), 7.49 ~ 7.56 (m, 2 H, ArH). 13 CNMR (400 Hz, DMSO) δ: 159.76, 154.46, 129.07, 123.61, 122.95, 120.72, 111.03, 102.41, 29.71, 27.79, 22.12, 14.05 See attached drawings 1-2. |
| Add 17.00kg (111.7mol, 1eq) of compound 6 to 34.00kg (2w/w) toluene, add 33.31kg (280.0mol, 2.5eq) of thionyl chloride, heat to 75~85℃, keep warm for 2~4 hour. After the reaction, the solvent was distilled off under reduced pressure at 65°C to obtain 18.99 kg (111.3 mol) of compound 7 as a colorless solution with a yield of about 97.4%. |
| Add 10.70kg (80.4mol, 1.0eq) of aluminum trichloride to 60.00kg of 1,2-dichloroethane at -20~-10℃, add 14.00kg (80.4mol, 1.0eq) of compound 5 under stirring, After stirring uniformly, 16.46kg (96.5mol, 1.2eq) of compound 7 is added at -20~-10°C, and the reaction is kept for 1~2 hours. After the reaction, the reaction solution was transferred to 90.00kg purified water, adjusted to pH 2 with dilute hydrochloric acid, separated into the organic phase and washed twice with 45.00kg purified water, the organic phase was washed with 45.00kg saturated sodium chloride aqueous solution, and 1.40kg was added. Dry with water sodium sulfate. The organic phase was concentrated under reduced pressure at 55°C to obtain 20.11 kg (65.0 mol) of compound 8. The yield is about 81.2%. |
| Add 20.00kg (64.9mol, 1.0eq) of compound 8 to 60.00kg (3w/w) of toluene, add 9.52kg (71.4mol, 1.1eq) of aluminum trichloride, and raise it to 80~90℃ to react for 2~4 hours . After the reaction, transfer the reaction solution to 90.00kg (4.5w/w) purified water, adjust the pH to 2 with dilute hydrochloric acid, separate the organic phase and wash 2 times with 50.00kg (2.5w/w) purified water, and add 50.00 for the organic phase kg (2.5w/w) saturated sodium chloride aqueous solution wash, add 2.00kg (0.1w/w) anhydrous sodium sulfate to dry. The organic phase was concentrated under reduced pressure at 65°C until a solid precipitated out, the temperature was lowered to 0°C, and the temperature was kept for 4 hours to crystallize. The wet product was filtered with suction and dried under vacuum at 80°C to obtain 16.60 kg (56.4 mol) of compound 9 as a white crystalline powder with a yield of about 86.9%. |
| 1 HNMR(400MHz,d DMSO )δ: 0.81~0.85(t,3H,-CH 3 ),1.24~1.29(m,2H,-CH 2 CH 3 ),1.68~1.70(m,2H,-CH 2 CH 2 CH 3 ),2.82~2.85(t,2H,Ar-CH 2 -CH 2 ), 6.91~7.72(m,8H,ArH), 10.46(m,1H,-OH). 13 CNMR(400 Hz,DMSO)δ:189.72,163.49,162.69,153.48,132.08,130.16,127.28,124.87,123.98 ,121.16,116.90,11 5.78,111.51,29.94,27.51,22.09,13.84. See attached drawings 3~4. |
| According to the operation of Example 1, the total yield was 46.6%. |
| Add 15.00kg (122.8mol, 1eq) of compound 1 and 30.82kg (147.4mol, 1.2eq) of compound 2 to 120.00kg (8w/w) ethyl acetate, add 10.19kg (73.7mol, 0.6eq) potassium carbonate, 1.00 kg (2.5 mol, 0.02 eq) methyl trioctyl ammonium chloride, stirred and heated to 75-85° C., reacted for 1 to 2 hours. After the reaction is completed, suction filtration, the filtrate is washed with 60.00kg (4w/w) purified water, and the organic phase is concentrated to dryness under reduced pressure at 40°C. Obtain 26.21kg (104.7mol) of compound 3, the yield is about 85.3%. |
| 26.00kg (103.9mol, 1eq) of compound 3 was added to 12.47kg (311.7mol, 3eq) of sodium hydroxide in 130.00kg (5w/w) methanol solution, and stirred at 20-30°C. After the reaction, it was concentrated to dryness under reduced pressure at 40°C. Add 130.00kg (5w/w) aqueous solution to dissolve, add 1N dilute hydrochloric acid to adjust the pH to 4, add 26.00kg (1w/w) ethyl acetate for extraction, add 26.00kg (1w/w) purified water for organic phase and wash once, organic phase Add 26.00kg (1w/w) saturated sodium chloride aqueous solution to wash, add 1.30kg (0.05w/w) anhydrous sodium sulfate to dry. The organic phase was concentrated to dryness under reduced pressure at 40°C. 23.50 kg (99.5 mol) of compound 4 was obtained, and the yield was about 95.8%. |
| Add 23.00kg (97.4mol, 1eq) of compound 4 to 184.00kg (8.0w/w) of acetic anhydride, add 64.40kg (2.8w/w) of sodium acetate, raise the temperature to 90~100℃ for reaction, and react for 1~2 hours . After the reaction, the reaction solution was transferred to 230.00kg (10w/w) purified water, stirred for 2 hours, and 69.00kg (3w/w) ethyl acetate was added for extraction. The organic phase was washed twice with 23.00kg (1.0w/w) purified water, the organic phase was washed with 23.00kg (1.0w/w) saturated sodium chloride aqueous solution, and 2.30kg (0.1w/w) was dried with anhydrous sodium sulfate. The organic phase was concentrated to dryness under reduced pressure at 40°C. 12.28kg (70.5mol) of compound 5 was obtained, and the yield was about 72.4%. |
| Add 15.00kg (98.6mol, 1eq) of compound 6 to 30.00kg (2w/w) of toluene, add 35.20kg (295.8mol, 3eq) of thionyl chloride, heat to 75~85℃, keep warm and react for 2~4 hours . After the reaction, the solvent was distilled off under reduced pressure at 65°C to obtain 16.63 kg (97.5 mol) of compound 7 as a colorless solution, with a yield of about 98.9%. |
| Add 27.56kg (206.7mol, 3.0eq) of aluminum trichloride to 60.00kg of 1,2-dichloroethane at -20~-10℃, add 12.00kg (68.9mol, 1.0eq) of compound 5 with stirring, After stirring uniformly, 14.11kg (82.7mol, 1.2eq) of compound 7 is added at -20~-10℃, and the reaction is kept for 1~2 hours. After the reaction, the reaction solution was transferred to 80.00kg purified water, adjusted to pH 2 with dilute hydrochloric acid, separated into the organic phase and washed twice with 40.00kg purified water, and the organic phase was washed with 40.00kg saturated sodium chloride aqueous solution, and 1.20kg was added. Dry with water sodium sulfate. The organic phase was concentrated under reduced pressure at 55°C to obtain 17.17 kg (55.7 mol) of compound 8, with a yield of about 80.8%. |
| Add 17.00kg (55.1mol, 1.0eq) of compound 8 to 51.00kg (3w/w) of toluene, add 22.04kg (165.3mol, 3.0eq) of aluminum trichloride, raise to 80~90℃ and react for 2~4 hours . After the reaction, the reaction solution was transferred to 76.50kg (4.5w/w) purified water, adjusted to pH 2 with dilute hydrochloric acid, separated, the organic phase was washed twice with 42.50kg (2.5w/w) purified water, and the organic phase was added 42.50kg (2.5w/w) saturated sodium chloride aqueous solution was washed, and 1.70kg (0.1w/w) anhydrous sodium sulfate was added for drying. The organic phase was concentrated under reduced pressure at 65°C until a solid precipitated out, the temperature was lowered to 0°C, and the temperature was kept for 4 hours to crystallize. After suction filtration, the wet product was dried in vacuum at 80° C. to obtain 13.81 kg (46.9 mol) of compound 9 as a white crystalline powder with a yield of about 85.1%. |
| According to the operation of Example 2, the total yield was 40.2%. |
| Add 15.00kg (122.8mol, 1eq) compound 1 and 30.82kg (147.4mol, 1.2eq) compound 2 to 120.00kg (8w/w) toluene, add 24.00kg (73.7mol, 0.6eq) cesium carbonate, 1.00kg (2.5mol, 0.02eq) methyl trioctyl ammonium chloride, stir and raise the temperature to 75~85℃, and react for 1~2 hours. After the reaction is completed, suction filtration, the filtrate is washed with 60.00kg (4w/w) purified water, and the organic phase is concentrated to dryness under reduced pressure at 40°C. 30.36 kg (121.3 mol) of compound 3 was obtained, and the yield was about 98.8%. |
| 30.00kg (120.0mol, 1eq) of compound 3 was added to 20.20kg (360.0mol, 3eq) of 150.00kg (5w/w) aqueous solution of potassium hydroxide, and stirred at 20-30°C. After the reaction, add 1N dilute hydrochloric acid to adjust the pH to 4, add 30.00kg (1w/w) ethyl acetate for extraction, add 30.00kg (1w/w) for the organic phase and wash once with purified water, and add 30.00kg (1w/w) for the organic phase Wash with saturated sodium chloride aqueous solution, add 1.50kg (0.05w/w) anhydrous sodium sulfate to dry. The organic phase was concentrated to dryness under reduced pressure at 40°C. 27.11 kg (114.7 mol) of compound 4 was obtained, and the yield was about 95.6%. |
| Add 27.00kg (114.3mol, 1eq) of compound 4 to 432.00kg (16.0w/w) of acetic anhydride, add 75.60kg (2.8w/w) of sodium acetate, raise the temperature to 90~100℃ for reaction, and react for 1~2 hours . After the reaction, the reaction solution was transferred to 540.00kg (20w/w) purified water, stirred for 5 hours, and 81.00kg (3w/w) ethyl acetate was added for extraction. The organic phase was washed twice with 27.00kg (1.0w/w) purified water, and the organic phase was washed with 27.00kg (1.0w/w) saturated aqueous sodium chloride solution, and dried with 2.70kg (0.1w/w) anhydrous sodium sulfate. The organic phase was concentrated to dryness under reduced pressure at 40°C. 14.57kg (83.6mol) of compound 5 was obtained, and the yield was about 73.1%. |
| Add 17.00kg (111.7mol, 1eq) of compound 6 to 34.00kg (2w/w) of toluene, add 35.20kg (295.8mol, 3eq) of thionyl chloride, heat to 75~85℃, keep the temperature and react for 2~4 hours . After the reaction, the solvent was distilled off under reduced pressure at 65°C to obtain 19.21 kg (112.6 mol) of compound 7 as a colorless solution, with a yield of about 100.0%. |
| Add 10.70kg (80.4mol, 1.0eq) of aluminum trichloride to 60.00kg of toluene at -20~-10℃, add 14.00kg (80.4mol, 1.0eq) of compound 5 with stirring, and after stirring, add at -20 16.46kg (96.5mol, 1.2eq) of compound 7 was added at ~-10°C, and the reaction was incubated for 1 to 2 hours. After the reaction, the reaction solution was transferred to 90.00kg purified water, adjusted to pH 2 with dilute hydrochloric acid, separated into the organic phase and washed twice with 45.00kg purified water, the organic phase was washed with 45.00kg saturated sodium chloride aqueous solution, and 1.40kg was added. Dry with water sodium sulfate. Add 10.72 kg (80.4 mol, 1.0 eq) of aluminum trichloride to the organic phase and raise it to 80-90° C. and react for 2 to 4 hours. After the reaction, the reaction solution was transferred to 90.00kg purified water, adjusted to pH 2 with dilute hydrochloric acid, separated into the organic phase and washed twice with 50.00kg purified water, the organic phase was washed with 50.00kg saturated sodium chloride aqueous solution, and 2.00kg Dry with water sodium sulfate. The organic phase was concentrated under reduced pressure at 65°C until a solid precipitated out, the temperature was lowered to 0°C, and the temperature was kept for 4 hours to crystallize. The wet product was filtered with suction and dried in vacuum at 80°C to obtain 14.47 kg (45.2 mol) of compound 9 as a white crystalline powder with a yield of 61.2%. |
| According to the operation of Example 3, the total yield was 42.3%. |
| Comparative Example 1 (CN104262304): |
| 700g (5.7mol, 1.0eq) of compound 1 was added to 2000g of ethyl acetate. After stirring, 1400g (6.7mol, 1.2eq) of compound 2 was slowly added. After the addition, 150g (0.46mol, 0.08eq) were added in sequence. Cesium carbonate and 50g (0.12mol, 0.02eq) methyl trioctyl ammonium chloride, and then slowly heated to 80 ℃, kept the reaction for 8 hours, at the end of the reaction, the ethyl acetate was evaporated under reduced pressure. After the evaporation, 2500g of toluene was added and controlled Warm to 20℃, continue to add 250g (4.6mol, 0.8eq) sodium methoxide, heat to reflux, keep the reaction for 5 hours; when the reaction is over, add dilute hydrochloric acid, adjust the pH to 7, stand still, separate the water phase; continue to add 600g Extract twice with water, discard the water phase, distill the organic phase under reduced pressure to remove the toluene, and collect the 135°C fraction. 383 g (2.2 mol) of compound 5 was obtained as a light yellow transparent liquid, and the yield was about 38.6%. |
| Add 360g (2.1mol, 1.0eq) of compound 5 to 2400g of toluene, heat to reflux, keep dehydration for 4 hours; after dehydration, cool to 15℃, add 420g (2.5mol, 1.2eq) of compound 7, 240g (1.8mol) , 0.86eq) zinc chloride, 60g (0.81mol, 0.4eq) N-nitrosodimethylamine, slowly increase the temperature to 80°C, keep the reaction for 10 hours; after the reaction is over, add dilute hydrochloric acid and adjust the pH to 1~2 , Continue to heat and stir for 2 hours, let stand, and discard the water phase; continue to add 450g of water to extract twice, discard the water phase, heat the organic phase to reflux, and keep dehydration for 4 hours; after dehydration, cool to 10°C, add 270g (2.1 mol, 1eq) Aluminum trichloride, slowly increase the temperature to 75°C, keep the reaction for 8 hours; after the reaction is over, add saturated sodium bicarbonate aqueous solution, adjust to pH 7, let stand, discard the water phase; add 360g saturated brine for extraction Three times, the water phase was discarded, the organic phase was distilled under reduced pressure, and it was steamed until a solid precipitated, and the temperature was reduced to 0°C, and the temperature was kept and stirred for 4 hours. After suction filtration, the wet product was vacuum dried at 80° C. for 8 hours to obtain 323 g (1.1 mol, 1 eq) of compound 9 as an off-white crystalline powder with a yield of about 52.4%. |
| According to the operation of Comparative Example 1, the total yield was 20.2%. |
| Comparative Example 2 (CN107382925): |
| 610g (5.0mol, 1.0eq) of compound 1 and 1728g (12.5mol, 2.5eq) of potassium carbonate were added to the mixture of 610kg (1w/w) DMF and 1830kg (3w/w) toluene, and heated to 60~ with stirring. Incubate at 70°C for half an hour, heat up to 80-100°C and add dropwise, 1098g (5.3mol, 1.05eq) of compound 2, react for 2 hours. After the reaction, 1830 g of water was added to wash twice, and the organic phase was concentrated to dryness under reduced pressure at 80°C. 1126 g (121.3 mol) of compound 3 was obtained, and the yield was about 90.1%. |
| 1100g (4.4mol, 1.0eq) of Compound 3 was added to 514g (4.8mol, 1.1eq) of trimethyl orthoformate and 7.6g (47mmol, 0.01eq) of p-toluenesulfonic acid in 11kg (10w/w) methanol solution, Stir at 20-30°C for 3 hours, add 2.4 g (44 mmol, 0.01 eq), and stir for 1 hour. Then it was concentrated to dryness under reduced pressure at 40°C. 1170 g (3.9 mol) of compound 0 was obtained, and the yield was about 89.7%. |
| Add 1100g (3.7mol, 1eq) of compound 0 to 2200g (2w/w) toluene, add 163g (4.1mol, 1.1eq) sodium hydroxide and 2200g (2w/w) water under stirring, 35~40 Stir and keep at ℃ for 4 hours. At the end of the reaction, 110g (0.1w/w) sodium chloride is added, and hydrochloric acid is added dropwise to adjust the pH to 1~2. The liquid was separated to obtain a toluene organic phase containing compound 4. Add 415g (4.1mol, 1.1eq) of triethylamine to the organic phase, raise it to 70~80℃ for reaction, add 782g (4.1mol, 1.1eq) of p-toluenesulfonyl chloride dropwise, and finish the reaction at 70~80℃ for 2 hours. A solution of 164g (4.1mol, 1.1eq) sodium hydroxide and 2200g (2w/w) water was added dropwise, and the reaction was kept at 70-80°C for 2 hours. Separate the liquids, wash the organic phase with 1100 g of 0.1M hydrochloric acid until neutral, and concentrate the organic phase to dryness at 80°C under reduced pressure. 453 g (2.6 mol) of compound 5 was obtained, and the yield was about 70.0%. |
| Add 409g (2.4mol, 1.0eq) compound 7, 336g (2.5mol, 1.05eq) aluminum trichloride to 60.00kg 1,2-dichloroethane at -20~-10℃, add 418g( 2.4 mol, 1.0 eq) Compound 5, incubated and reacted for 4 hours. Add 320g (2.4mol, 1.0eq) of aluminum trichloride to the organic phase, raise to reflux, and react under reflux for 6 hours. After the reaction, the reaction solution was transferred to 1000g purified water, the organic phase was separated and washed twice with 500g purified water, and the organic phase was purified by column chromatography to obtain 283g (1.0mol) of compound 9 as white crystalline powder with a yield of about 40.1%. . |
| According to the operation of Comparative Example 2, the total yield was 22.7%. |
| Description of the drawings |
| Figure 1 shows the proton nuclear magnetic resonance spectrum of compound 5; |
| Figure 2 shows the carbon nuclear magnetic resonance spectrum of compound 5; |
| Figure 3 shows the proton nuclear magnetic resonance spectrum of compound 9; |
| Figure 4 shows the carbon nuclear magnetic resonance spectrum of compound 9. |


PATENT
CN106946822
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN207044243&tab=PCTDESCRIPTION&_cid=P11-KL0B7U-08304-1

| Example 1: Preparation of 2-butyl-3-(4-hydroxybenzoyl)benzofuran |
| Step (1): Take 117.1g (0.5mol) of 1-(4-methoxyphenyl)-1,3-heptanedione and 56.1g (0.5mol) of acrolein dimer in organic solvent tetrahydrofuran (2L). ), 126.9 g (0.5 mol) of elemental iodine and 3.4 g (25 mmol) of zinc chloride were added, and the above reaction solution was stirred and reacted in a reactor equipped with magnetic stirring at 40°C for 2 hours. After the reaction, the acidic reaction system was neutralized to neutrality with saturated sodium thiosulfate and saturated sodium bicarbonate respectively; the aqueous phase obtained by liquid-liquid extraction with ethyl acetate; the organic phase was combined and concentrated under reduced pressure; ethyl acetate and Diethyl ether was recrystallized and separated to obtain 98.6 g (0.32 mol) of 2-butyl-3-(4-methoxybenzoyl)benzofuran. 1 H NMR(400MHz, CDCl 3 , TMS, 25℃) δ = 7.84 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.2 Hz, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.26 (dd, J = 9.4,5.9Hz,1H), 7.18(t,J=7.5Hz,1H), 6.96(d,J=8.8Hz,2H), 3.89(s,3H), 2.91(t,J=7.6Hz,2H) ,1.80–1.71(m,2H),1.36(dd,J=15.0,7.4Hz,2H), 0.89ppm(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=190.7,164.8,163.6,153.8,132.1,131.8,127.4,124.3,123.4,121.4,116.9,113.8,111.1,55.6,30.3,28.0,22.5,13.8ppm |
 |
| Step (2): Put 98.6g (0.32mol) of 2-butyl-3-(4-methoxybenzoyl)benzofuran obtained in step (1) into a reactor equipped with magnetic stirring. 2.1g (16mmol) of aluminum oxide was dissolved in 2L of acetonitrile; the mixture was stirred and reacted at 80°C for 5 hours. After the reaction, the acidic reaction system was neutralized with saturated sodium bicarbonate solution to neutrality; The aqueous phase obtained by extraction; the organic phases were combined and concentrated under reduced pressure; ethyl acetate and ether were recrystallized and separated to obtain 52.9 g (0.18 mol) of the 2-butyl-3-(4-hydroxybenzoyl)benzofuran. 1 H NMR(400MHz, CDCl 3 ,TMS,25℃)δ=10.46(s,1H),7.68(d,J=8.6Hz,2H), 7.62(d,J=8.1Hz,1H), 7.33(dd,J=15.7,7.9Hz, 2H), 7.24 (t, J = 7.4 Hz, 1H), 6.89 (d, J = 8.6 Hz, 2H), 2.80 (t, J = 7.5 Hz, 2H), 1.65 (dt, J = 15.0, 7.5 Hz, 2H),1.23(dd,J=14.7,7.3Hz,2H),0.80(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=189.3,163.1,162.2,153.0,131.6,129.7,126.8,124.5,123.6,120.7,116.4,115.3,111.1,29.5,27.1,21.6,13.4ppm. |
 |
| The yield of Example 1 was 36%, wherein the yield of step (1) was 64%, and the yield of step (2) was 56%. |
| Example 2: Preparation of 2-butyl-3-(4-hydroxybenzoyl)benzofuran |
| Step (1): Take 58.5g (0.25mol) of 1-(4-methoxyphenyl)-1,3-heptanedione and 56.1g (0.5mol) of acrolein dimer in the organic solvent methylene chloride (2L), then add 165.8g (0.5mol) of carbon tetrabromide, BBr 3 6.26g (25mmol), the above reaction solution was stirred and reacted for 4 hours at 25°C in a reactor equipped with magnetic stirring. After the reaction, the acidic reaction system was neutralized with saturated sodium bicarbonate; the aqueous phase obtained by liquid-liquid extraction with dichloromethane; the organic phases were combined and concentrated under reduced pressure; ethyl acetate and ether were recrystallized to obtain 2-butane Benzyl-3-(4-methoxybenzoyl)benzofuran 49.3 g (0.16 mol). 1 H NMR(400MHz, CDCl 3 , TMS, 25℃) δ = 7.84 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.2 Hz, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.26 (dd, J = 9.4,5.9Hz,1H), 7.18(t,J=7.5Hz,1H), 6.96(d,J=8.8Hz,2H), 3.89(s,3H), 2.91(t,J=7.6Hz,2H) ,1.80–1.71(m,2H),1.36(dd,J=15.0,7.4Hz,2H), 0.89ppm(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=190.7,164.8,163.6,153.8,132.1,131.8,127.4,124.3,123.4,121.4,116.9,113.8,111.1,55.6,30.3,28.0,22.5,13.8ppm |
 |
| Step (2): Put 49.3g (0.16mol) of 2-butyl-3-(4-methoxybenzoyl)benzofuran obtained in step (1) into a reactor equipped with magnetic stirring. 1.1g (8mmol) of boron diethyl ether was dissolved in 2L of 1,2-dichloroethane; the mixture was stirred and reacted at 60℃ for 5 hours. After the reaction, the acidic reaction system was neutralized with saturated sodium bicarbonate solution The aqueous phase obtained by liquid-liquid extraction with ethyl acetate; the organic phases were combined and concentrated under reduced pressure; ethyl acetate and ether were recrystallized and separated to obtain the 2-butyl-3-(4-hydroxybenzoyl) benzo Furan 32.37g (0.11mol). 1 H NMR(400MHz, CDCl 3 ,TMS,25℃)δ=10.46(s,1H),7.68(d,J=8.6Hz,2H), 7.62(d,J=8.1Hz,1H), 7.33(dd,J=15.7,7.9Hz, 2H), 7.24 (t, J = 7.4 Hz, 1H), 6.89 (d, J = 8.6 Hz, 2H), 2.80 (t, J = 7.5 Hz, 2H), 1.65 (dt, J = 15.0, 7.5 Hz, 2H),1.23(dd,J=14.7,7.3Hz,2H),0.80(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=189.3,163.1,162.2,153.0,131.6,129.7,126.8,124.5,123.6,120.7,116.4,115.3,111.1,29.5,27.1,21.6,13.4ppm. |
 |
| The yield of Example 2 was 44%, wherein the yield of step (1) was 64%, and the yield of step (2) was 68%. |
| Example 3: Preparation of 2-butyl-3-(4-methylbenzoyl)benzofuran |
| Step (1): Take 117.1g (0.5mol) of 1-(4-methoxyphenyl)-1,3-heptanedione, and dissolve 56.1g (0.5mol) of acrolein dimer in organic solvent ethanol (2L) ), then add 66.8g (0.5mol) of N-chlorosuccinimide, ZrCl 4 5.8 g (25 mmol), the above reaction solution was stirred and reacted in a reactor equipped with magnetic stirring at 70°C for 8 hours. After the reaction is completed, the acidic reaction system is neutralized with saturated sodium bicarbonate; the aqueous phase obtained by liquid-liquid extraction with ethyl acetate; the organic phases are combined and concentrated under reduced pressure; ethyl acetate and ether are recrystallized and separated to obtain 2-butane 104.7 g (0.34 mol) of phenyl-3-(4-methoxybenzoyl)benzofuran. 1 H NMR(400MHz, CDCl 3 , TMS, 25℃) δ = 7.84 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.2 Hz, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.26 (dd, J = 9.4,5.9Hz,1H), 7.18(t,J=7.5Hz,1H), 6.96(d,J=8.8Hz,2H), 3.89(s,3H), 2.91(t,J=7.6Hz,2H) ,1.80–1.71(m,2H),1.36(dd,J=15.0,7.4Hz,2H), 0.89ppm(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=190.7,164.8,163.6,153.8,132.1,131.8,127.4,124.3,123.4,121.4,116.9,113.8,111.1,55.6,30.3,28.0,22.5,13.8ppm |
 |
| Step (2): Put 104.7g (0.34mol) of 2-butyl-3-(4-methoxybenzoyl)benzofuran obtained in step (1) into a reactor equipped with magnetic stirring. 6.5g (34mmol) of boron chloride was dissolved in 2L acetonitrile; the mixture was stirred and reacted at 0°C for 1 hour. After the reaction, the acidic reaction system was neutralized with saturated sodium bicarbonate solution to neutrality; The aqueous phase obtained by extraction; the organic phases are combined and concentrated under reduced pressure; ethyl acetate and ether are recrystallized and separated to obtain 79.4 g (0.27 mol) of the 2-butyl-3-(4-hydroxybenzoyl)benzofuran. 1 H NMR(400MHz, CDCl 3 ,TMS,25℃)δ=10.46(s,1H),7.68(d,J=8.6Hz,2H), 7.62(d,J=8.1Hz,1H), 7.33(dd,J=15.7,7.9Hz, 2H), 7.24 (t, J = 7.4 Hz, 1H), 6.89 (d, J = 8.6 Hz, 2H), 2.80 (t, J = 7.5 Hz, 2H), 1.65 (dt, J = 15.0, 7.5 Hz, 2H),1.23(dd,J=14.7,7.3Hz,2H),0.80(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=189.3,163.1,162.2,153.0,131.6,129.7,126.8,124.5,123.6,120.7,116.4,115.3,111.1,29.5,27.1,21.6,13.4ppm. |
 |
| The yield of Example 3 was 54%, wherein the yield of step (1) was 68%, and the yield of step (2) was 79%. |
| Example 4: Preparation of 2-butyl-3-(4-hydroxybenzoyl)benzofuran |
| Step (1): Take 117.1g (0.5mol) of 1-(4-methoxyphenyl)-1,3-heptanedione, and dissolve 112.0g (1.0mol) of acrolein dimer in organic solvent toluene (2L) ), then add 142.9g (0.5mol) of dibromoglycine, AlCl 3 5.8 g (50 mmol), the above reaction liquid was stirred and reacted in a reactor equipped with magnetic stirring at 100°C for 1 hour. After the reaction is completed, the acidic reaction system is neutralized with saturated sodium bicarbonate; the aqueous phase obtained by liquid-liquid extraction with ethyl acetate; the organic phases are combined and concentrated under reduced pressure; ethyl acetate and ether are recrystallized and separated to obtain 2-butane Group-3-(4-methoxybenzoyl)benzofuran 110.9g (0.36mol). 1 H NMR(400MHz, CDCl 3 , TMS, 25℃) δ = 7.84 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.2 Hz, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.26 (dd, J = 9.4,5.9Hz,1H), 7.18(t,J=7.5Hz,1H), 6.96(d,J=8.8Hz,2H), 3.89(s,3H), 2.91(t,J=7.6Hz,2H) ,1.80–1.71(m,2H),1.36(dd,J=15.0,7.4Hz,2H), 0.89ppm(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=190.7,164.8,163.6,153.8,132.1,131.8,127.4,124.3,123.4,121.4,116.9,113.8,111.1,55.6,30.3,28.0,22.5,13.8ppm |
 |
| Step (2): Put 110.9g (0.36mol) of 2-butyl-3-(4-methoxybenzoyl)benzofuran obtained in step (1) into a reactor equipped with magnetic stirring. 10.2g (72mmol) of boron diethyl ether was dissolved in 2L acetonitrile; the mixture was stirred and reacted at 80°C for 2 hours. After the reaction, the acidic reaction system was neutralized with saturated sodium bicarbonate solution to neutrality; The aqueous phase obtained by liquid extraction; the organic phases were combined and concentrated under reduced pressure; ethyl acetate and ether were recrystallized and separated to obtain the 2-butyl-3-(4-hydroxybenzoyl)benzofuran 82.3g (0.28mol) . 1 H NMR(400MHz, CDCl 3 ,TMS,25℃)δ=10.46(s,1H),7.68(d,J=8.6Hz,2H), 7.62(d,J=8.1Hz,1H), 7.33(dd,J=15.7,7.9Hz, 2H), 7.24 (t, J = 7.4 Hz, 1H), 6.89 (d, J = 8.6 Hz, 2H), 2.80 (t, J = 7.5 Hz, 2H), 1.65 (dt, J = 15.0, 7.5 Hz, 2H),1.23(dd,J=14.7,7.3Hz,2H),0.80(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=189.3,163.1,162.2,153.0,131.6,129.7,126.8,124.5,123.6,120.7,116.4,115.3,111.1,29.5,27.1,21.6,13.4ppm. |
 |
| The yield of Example 4 was 56%, wherein the yield of step (1) was 72%, and the yield of step (2) was 78%. |
| Example 5: Preparation of 2-butyl-3-(4-methylbenzoyl)benzofuran |
| Step (1): Take 117.1g (0.5mol) of 1-(4-methoxyphenyl)-1,3-heptanedione, and dissolve 28g (0.25mol) of acrolein dimer in the organic solvent dichloromethane ( 2L), then add 159.8g (0.5mol) of liquid bromine, ZnCl 2 6.8 g (50 mmol), the above reaction solution was stirred and reacted for 8 hours at 25°C in a reactor equipped with magnetic stirring. After the reaction is completed, the acidic reaction system is neutralized with saturated sodium bicarbonate; the aqueous phase obtained by liquid-liquid extraction with ethyl acetate; the organic phases are combined and concentrated under reduced pressure; ethyl acetate and ether are recrystallized and separated to obtain 2-butane Group-3-(4-methoxybenzoyl)benzofuran 58.5g (0.19mol). 1 H NMR(400MHz, CDCl 3 , TMS, 25℃) δ = 7.84 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.2 Hz, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.26 (dd, J = 9.4,5.9Hz,1H), 7.18(t,J=7.5Hz,1H), 6.96(d,J=8.8Hz,2H), 3.89(s,3H), 2.91(t,J=7.6Hz,2H) ,1.80–1.71(m,2H),1.36(dd,J=15.0,7.4Hz,2H), 0.89ppm(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=190.7,164.8,163.6,153.8,132.1,131.8,127.4,124.3,123.4,121.4,116.9,113.8,111.1,55.6,30.3,28.0,22.5,13.8ppm |
 |
| Step (2): Put 58.5g (0.19mol) of 2-butyl-3-(4-methoxybenzoyl)benzofuran obtained in step (1) into a reactor equipped with magnetic stirring. 7.2g (38mmol) of sulfonic acid was dissolved in 2L of toluene; the mixture was stirred and reacted at 100°C for 4 hours. After the reaction, the acidic reaction system was neutralized with saturated sodium bicarbonate solution to neutrality; The aqueous phase obtained by extraction; the organic phases were combined and concentrated under reduced pressure; ethyl acetate and ether were recrystallized and separated to obtain 41.2 g (0.14 mol) of the 2-butyl-3-(4-hydroxybenzoyl)benzofuran. 1 H NMR(400MHz, CDCl 3 ,TMS,25℃)δ=10.46(s,1H),7.68(d,J=8.6Hz,2H), 7.62(d,J=8.1Hz,1H), 7.33(dd,J=15.7,7.9Hz, 2H), 7.24 (t, J = 7.4 Hz, 1H), 6.89 (d, J = 8.6 Hz, 2H), 2.80 (t, J = 7.5 Hz, 2H), 1.65 (dt, J = 15.0, 7.5 Hz, 2H),1.23(dd,J=14.7,7.3Hz,2H),0.80(t,J=7.4Hz,3H). 13 C NMR(100MHz,CDCl 3 ,25℃)δ=189.3,163.1,162.2,153.0,131.6,129.7,126.8,124.5,123.6,120.7,116.4,115.3,111.1,29.5,27.1,21.6,13.4ppm. |
 |
| The yield of Example 5 was 28%, wherein the yield of step (1) was 38%, and the yield of step (2) was 74% |
| In addition to the specific types of halogenated reagents, acid catalysts, and organic solvent raw materials used in the above embodiments, other halogenated reagents, acid catalysts, and organic solvents can also be used; wherein, the halogenated reagent in step (1) is preferably Liquid bromine, elemental iodine, N-iodosuccinimide, N-bromosuccinimide, N-chlorosuccinimide, 1,3-dichloro-5,5-dimethyl Any of hydantoin, dibromohydantoin, bromochlorohydantoin, carbon tetrabromide, and the acid catalyst is boron tribromide, boron trifluoride ether, aluminum chloride, hydrogen fluoride, zinc chloride, zirconium chloride At least one of the organic solvents is dichloromethane, 1,2-dichloroethane, acetonitrile, tetrahydrofuran, ethanol; the acid catalyst in step (2) is boron tribromide, trifluoride One of boron diethyl ether, aluminum chloride, hydrogen fluoride, sulfuric acid, and p-toluenesulfonic acid, and the organic solvent is any of methylene chloride, 1,2-dichloroethane, acetonitrile, tetrahydrofuran, ethanol, and toluene . The specific types of acid catalysts and organic solvents used in steps (1) and (2) of the present invention may be the same or different; in addition, the boiling point of the organic solvent used must be lower than the corresponding treatment temperature. |
| Unless otherwise specified, the various reaction raw materials in the present invention (eg, acrolein dimer, 1-(4-methoxyphenyl)-1,3-heptanedione, etc.) are commercially available The purity is preferably analytically pure. |
PATENT
CN109988131
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN248953969&_cid=P11-KL0BBI-08827-1
| Amiodarone (Amiodarone), also known as amiodarone, was first introduced as a coronary artery dilator, and Rosenbanm was the first to use it in the treatment of anti-arrhythmia in 1976. Amiodarone is extremely toxic. The lethal dose of intravenous injection is 10 times that of the therapeutic dose. The large oral lethal dose is negligible. Long-term larger doses are safe. Amiodarone hydrochloride (ADHC) is the hydrochloride of amiodarone, which was first marketed in Italy in 1984, and its structure is as follows: |
 |
| Amiodarone hydrochloride is a class III antiarrhythmic drug. It is mainly used clinically for supraventricular and ventricular tachyarrhythmias. It is also used for various organic heart diseases and acute coronary syndromes. It can be used as a symptomatic The first-line treatment of atrial fibrillation with left ventricular insufficiency or chronic heart failure. At present, it has become the drug of choice for the prevention of AMI with ventricular tachyarrhythmia, post-infarction ventricular arrhythmia, heart failure with arrhythmia, and sudden cardiac death. |
| In the existing synthetic methods, amiodarone hydrochloride is all obtained by etherification and salt formation with 2-butyl-(4-hydroxy-3,5-diiodobenzoyl)benzofuran as the key intermediate. The structure of this key intermediate is as follows: |
| As a key intermediate of amiodarone hydrochloride, its synthesis is relatively mature. The traditional synthetic route has been applied to industrial production. The route is as follows: |
| This process has the following problems: 1. Long route, complicated steps, and various operations; 2. Low yield and high cost; 3. Many wastes and high waste liquid treatment cost. Therefore, this route is no longer suitable for the current industrial and environmental protection requirements, and more efficient and green methods need to be developed. |
| Patent CN104262304 and CN1858042 improved the above synthesis method and developed a new synthesis method, the route is as follows: |
| This method has been greatly improved compared with the earlier process, but the yield and purity of 2-butylbenzofuran prepared by the route method are low, which leads to a greatly reduced yield and purity of amiodarone hydrochloride. CN107382925 continues to improve the above process, and the purity and yield have increased, but column chromatography purification is required, which is not suitable for industrial large-scale production. |
| 1. Preparation of intermediate 2-butylbenzofuran: |
| Put 5.5 g K 2 CO 3 , 076 g of CuI and 0.74 g of TBAI were added to a 100 ml reaction flask containing 30 ml of toluene, and 4.4 g of 2-iodophenol, 2.5 ml of 1-hexyne and 60 mg of nickel catalyst were added to it, and replaced with nitrogen 3 Secondly, the reaction was kept at 50°C and stirred for 18-22 hours under nitrogen protection. The reaction solution was filtered, and the filtrate was washed with 40 ml of 5% NaOH aqueous solution and twice with 40 ml of water. The filtrate was concentrated under reduced pressure to obtain 3.12 g of a dark yellow solid, namely 2-butylbenzofuran, with a yield of 89.7%. |
| 2. Synthesis of intermediate 2-butyl-(4-methoxybenzoyl)benzofuran: |
| Add 5.50 g of aluminum trichloride and 24 ml of dichloromethane to a 100 ml reaction flask, stir and lower the temperature to 0°C, and add 7.0 g of p-methoxybenzoyl chloride dropwise to it within 5°C, and keep warm after dropping. Stir for 1 hour. Dissolve 6.00 g of 2-butylbenzofuran in 24 ml of dichloromethane and add dropwise to the above reaction solution within 5°C of temperature control. After dropping, slowly raise the temperature to 25°C, keep the temperature for 2 hours, and complete the reaction. After cooling to room temperature, it was poured into ice water for separation, the aqueous phase was extracted with dichloromethane, the organic layers were combined, washed twice with water, and the organic phase was concentrated under reduced pressure to obtain 10.60 g of oil. |
| 3. Synthesis of 2-butyl-(4-hydroxybenzoyl)benzofuran: |
| Add 10.00 g of 2-butyl-(4-methoxybenzoyl) benzofuran, 4.50 g of aluminum trichloride and 40 ml of toluene into a 100 ml reaction flask. The temperature is raised to reflux, and the reaction is kept warm for 6 hours. The reaction is complete . Cool down to 0℃, pour into ice water, separate the liquids, extract the aqueous phase with toluene, combine the organic phases, add equal volumes of water, adjust the pH to above 12 with NaOH solution, separate the liquids, adjust the pH to less than 3 with hydrochloric acid for the aqueous phase , Filtered, and the filter cake was vacuum dried to obtain 8.16 g of light yellow solid. |
| 4. Synthesis of 2-butyl-(4-hydroxy-3,5-diiodobenzoyl)benzofuran: |
| Add 8 g of 2-butyl-(4-hydroxybenzoyl) benzofuran, 15.18 g of iodine, 8.26 g of potassium carbonate and 48 ml of ethanol to a 100 ml reaction flask, and heat to reflux with stirring, and keep the reaction temperature for 2 hours. The reaction is over. The temperature was lowered to room temperature, filtered, the filtrate was added dropwise to the sodium metabisulfite aqueous solution, after dripping, the mixture was kept and stirred for 0.5 hours, filtered, the filter cake was washed twice with water, and the solid was vacuum dried to obtain 14.61 g of off-white solid. |
| 5. Synthesis of 2-butyl-[4-[2-(diethylamino)hydroxyethyl]-3,5-diiodobenzoyl]benzofuran: |
| Add 12.00 g of 2-butyl-(4-hydroxy-3,5-diiodobenzoyl) benzofuran and 120 ml of toluene into a 250 ml reaction flask. The temperature is raised to 60°C. After the solid is dissolved, add to it 4.94 g of 2-diethylaminochloroethane hydrochloride, 5.65 g of potassium carbonate and 8.50 g of water were heated to reflux, stirred and reacted for 8 hours, and the reaction was completed. The reaction solution was washed 3 times with water, 0.60 g activated carbon was added to the organic phase, the temperature was raised to reflux and stirred for 1 hour, and then filtered with suction. The filtrate was concentrated under reduced pressure until solids began to precipitate, and the temperature was reduced to 0°C for crystallization. The cold toluene was washed twice, and the solid was vacuum-dried at 80°C to obtain 13.97 g of a finished white solid, which was the finished product of amiodarone hydrochloride. |
| Compared with the existing production process, the preparation method of amiodarone hydrochloride in this embodiment simplifies the operation and improves the convenience of operation and the stability of the product. Through the control of the catalyst and material ratio, the purity and yield of each intermediate are improved, and the product does not require column chromatography to purify, which saves costs and improves production efficiency, which provides convenience for industrial large-scale production. |
| 1. Preparation of intermediate 2-butylbenzofuran: |
| Put 5.5 g K 2 CO 3 , 0.76 g of CuI and 0.74 g of TBAI were added to a 100 ml reaction flask containing 30 ml of toluene, and 4.4 g of 2-iodophenol, 2.5 ml of 1-hexyne and 190 mg of ruthenium catalyst were added to it, and replaced with nitrogen 3 Secondly, the reaction was kept at 50°C and stirred for 22-28 hours under nitrogen protection. The reaction solution was filtered, and the filtrate was washed with 40 ml of 5% NaOH aqueous solution and 40 ml of water twice. The filtrate was concentrated under reduced pressure to obtain 3.25 g of a dark yellow solid, which was 2-butylbenzofuran, with a yield of 93.4%. |
| The remaining steps are the same as in Example 1. |
| 1. Preparation of intermediate 2-butylbenzofuran: |
| Put 5.5 g K 2 CO 3 , 0.76 g of CuI and 0.74 g of TBAI were added to a 100 ml reaction flask containing 30 ml of toluene, and 4.4 g of 2-iodophenol, 2.5 ml of 1-hexyne and 89 mg of palladium catalyst were added to it, and replaced with nitrogen 3 Secondly, the reaction was kept at 40°C under nitrogen and stirred for 26-30 hours. The reaction solution was filtered, and the filtrate was washed with 40 ml of 5% NaOH aqueous solution and 40 ml of water twice. The filtrate was concentrated under reduced pressure to obtain 3.36 g of a dark yellow solid, which was 2-butylbenzofuran, with a yield of 96.6%. |
| The remaining steps are the same as in Example 1. |
| 1. Preparation of intermediate 2-butylbenzofuran: |
| Put 5.5 g K 2 CO 3 , 0.76 g of CuI and 0.74 g of TBAI were added to a 100 ml reaction flask containing 30 ml of toluene, and 4.4 g of 2-iodophenol, 2.5 ml of 1-hexyne and 100 mg of rhodium catalyst were added to it, and replaced with nitrogen 3 Next, the reaction was kept at 40°C under nitrogen protection and stirred for 20-24 hours. The reaction solution was filtered, and the filtrate was washed with 40 ml of 5% NaOH aqueous solution and 40 ml of water twice. The filtrate was concentrated under reduced pressure to obtain 2.93 g of dark yellow solid, which is 2-butylbenzofuran, with a yield of 84.2%. |
| The remaining steps are the same as in Example 1. |
| 1. Preparation of intermediate 2-butylbenzofuran: |
| Put 5.5 g K 2 CO 3 , 0.76 g of CuI and 0.74 g of TBAI were added to a 100 ml reaction flask containing 30 ml of toluene, and 4.4 g of 2-iodophenol, 2.5 ml of 1-hexyne and 80 mg of gold catalyst were added to it, and replaced with nitrogen 3 Secondly, the reaction was kept at 40°C and stirred for 20-28 hours under nitrogen protection. The reaction solution was filtered, and the filtrate was washed with 40 ml of 5% NaOH aqueous solution and 40 ml of water twice. The filtrate was concentrated under reduced pressure to obtain 3.07 g of dark yellow solid, which is 2-butylbenzofuran, with a yield of 88.2%. |
|
The remaining steps are the same as in Example 1. |
?///////////



















![[1860-5397-7-57-i76]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i76.png?max-width=550&background=EEEEEE)