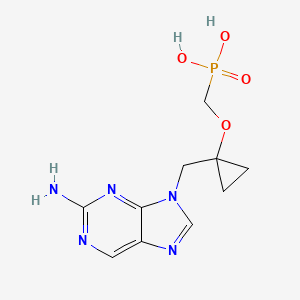
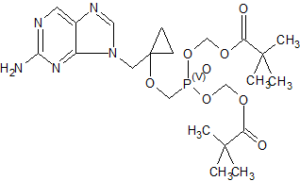Besifovir
- Molecular FormulaC10H14N5O4P
- Average mass299.223 Da
- UNII-4PLG22CQUU
Besifovir (INN) is an investigational medication to treat hepatitis B virus (HBV) infection. It is a novel and potent acyclic nucleotide phosphonate with a similar chemical structure to adefovir and tenofovir.[2]
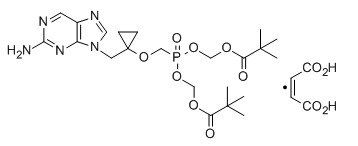
Besifovir dipivoxil maleate
CAS:1039623-01-2, Propanoic acid, 2,2-dimethyl-, 1,1′-[[[[[1-[(2-amino-9H-purin-9-yl)methyl]cyclopropyl]oxy]methyl]phosphinylidene]bis(oxymethylene)] ester, (2Z)-2-butenedioate (1:1)
| Molecular Formula | C22 H34 N5 O8 P . C4 H4 O4 |
| Molecular Weight | 643.58 |
| Highest Phase | Launched – 2017, korea
Besifovir dipivoxil maleate |
Besifovir, also known as ANA-380; LB-80380; PMCDG dipivoxil, is a reverse transcriptase inhibitor potentially for treatment of hepatitis B infection. LB80380 is a prodrug and an oral nucleotide analogue that inhibits viral replication by incorporation into the viral DNA. Antiviral activity against wild-type virus and virus with drug-resistant mutations was demonstrated in Phase II trials, with significant reduction of viral load in patients treated with LB80380. LB80380 was also shown to be safe and well tolerated.
CAS 441785-26-8
Chemical Formula: C22H34N5O7P
Molecular Weight: 511.5158
Besifovir; ANA-380; AN-380; ANA 380; LB-80380; LB 80380; LB80380; PMCDG dipivoxil.
IUPAC/Chemical Name: ((((1-((2-amino-9H-purin-9-yl)methyl)cyclopropoxy)methyl)phosphanediyl)bis(oxy))bis(methylene) bis(2,2-dimethylpropanoate)
- By Constance Williams
- Published 2017.10.26 16:51
- Updated 2017.10.26 19:12
|
Ildong Pharmaceutical will release its first chronic hepatitis B therapy of nucleotide series “Besivo” as an insurance benefit drug next month, the company said Thursday. Besivo is a treatment for chronic hepatitis B based on the nucleotide sequence, which is composed of besifovir. The price of the insurance is 3,403 won ($3.02) per tablet, which was recently confirmed by the Ministry of Health and Welfare보건복지부. Insurance benefits also cover El-carnitine medications used in combination, and the insurance price for one tablet (330mg) is 111 won. According to the results of clinical trials, Besivo has demonstrated comparable levels of therapeutic efficacy in a randomized, double-blind trial compared with traditional therapies such as Entecavir (trade name: Baraclude) and Tenofovir (trade name: Viread). Besivo improved the prospects as a valid option for the treatment of chronic hepatitis B by improving the side effects found in the existing medications. In particular, further analysis of clinical trials has shown that typical side effects such as decreased renal function and decreased bone density, which was a problem in the existing Tenofovir. Knodell necro-inflammatory score, was also superior to the control group regarding the histological improvement of the liver.
For the deterioration of renal function, the rate of increase in serum creatinine — a test that measures kidney function – was significantly lower than that of Tenofovir. With the case of measuring bone mineral density, the proportion of patients showing bone turnover increased and the percentage of patients showing average bone mineral density decreased in the case of Tenofovir. With Besivo, the rate of patients with bone loss decreased, and the percentage of patients with average bone mineral density increased. Ildong Pharma 일동제약 (CEO: Yun Woong-sup윤웅섭) plans to go on marketing with the idea that Besivo is a domestic drug that secures safety by improving side effects of existing medicines as well as treatment effects that are comparable to that of foreign pharmaceutical companies. In particular, the company expects the cost of pharmaceuticals to be 25 percent lower than that of Viread, the leading drug in the market. “Due to the nature of chronic hepatitis B treatment for long-term use, safety is critical, and there are few side effects, so Besivo is highly valuable as a few nucleotide drugs existing in consideration of cross-resistance,” said Professor Ahn Sang-hoon안상훈 of Severance Hospital세브란스병원who participated in the clinical study. “There is a strong competitive edge regarding the advantages of Besivo in connection with the entry into the Asian market, where the demand for therapeutic drugs is increasing as a major outbreak of hepatitis B,” he added. |
Il Dong, under license from LG Life Sciences , has developed and launched Besivo (besifovir dipivoxil maleate), a phosphonate nucleoside inhibitor of HBV polymerase, for treating HBV infection. In October 2012, Il Dong was planning on seeking to outlicense the drug outside of Korea.
Besifovir dipivoxil maleate is a DNA polymerase inhibitor discovered and developed by LG Chem. The product was launched in Korea in 2017 by codeveloper ILDONG for the treatment of hepatitis B.
In April 2004, Anadys (acquired by Roche in 2011) obtained an exclusive license from LG Chem for the commercialization of LB-80380 worldwide excluding China, Korea, India and Southeast Asia. In August 2007, Anadys reported that it had discontinued development of LB-80380 and returned all rights to LG Chem in order to focus on other key compounds.
PAPER
A Novel Class of Phosphonate Nucleosides. 9-[(1-Phosphonomethoxycyclopropyl)methyl]guanine as a Potent and Selective Anti-HBV Agent
Abstract

9-[1-(Phosphonomethoxycyclopropyl)methyl]guanine (PMCG, 1), representative of a novel class of phosphonate nucleosides, blocks HBV replication with excellent potency (EC50 = 0.5 μM) in a primary culture of HepG2 2.2.15 cells. It exhibits no significant cytotoxicity in several human cell lines up to 1.0 mM. It does not inhibit replication of human immunodeficiency virus (HIV-1) or herpes simplex virus (HSV-1) at 30 μM. Many purine base analogues of 1 also exhibit inhibitory activity against HBV, but at 30 μM, pyrimidine analogues do not. 1 is 4 times more potent than 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA), which was used as a positive control (EC50 = 2.0 μM). The characteristic cyclopropyl moiety at the 2‘-position of 1 was prepared by titanium-mediated Kulinkovich cyclopropanation. 1 was modified to give the orally available drug candidate, PMCDG Dipivoxil (2). Compound 2 exhibited excellent efficacy when administered at 5 mg per kg per day in a study with woodchucks infected with woodchuck hepatitis B virus (WHBV). Drug candidate 2 has successfully completed phase I clinical trials and is currently undergoing phase II clinical studies for evaluation of efficacy.
({1-[(2-Amino-9H-purin-9-yl)methyl]cyclopropyl}oxy)methylphosphonic Acid (PMCDG, 8). 8 (89.5% yield) as yellowish solids. The compound was recrystallized from water for X-ray crystallography. 1H NMR (400 MHz, DMSO-d6): δ 0.92 (br q, 4H), 3.76 (d, J = 12.0 Hz, 2H), 4.33 (s, 2H), 8.0 (br s, 2H), 8.74 (s, 1H), 9.00 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 11.6 (2C), 45.9, 62.9 (d, J = 15.0 Hz), 63.0 (d, J = 161 Hz), 125.6, 139.1, 149.8, 154.2, 157.1. HRMS (MH+): 300.0862 calcd for C10H14N5O4P, found 300.0872. Anal. (C10H14N5O4P·H2O) C, H, N.
({1-[(2-Amino-9H-purin-9-yl)methyl]cyclopropyl}oxy)methylphosphonic Acid Dipivoxyl (PMCDG Dipivoxyl, 2). 2 (38.5% yield) as white solids. mp: 92 °C. 1H NMR (400 MHz, CDCl3): δ 0.89 (br t, 2H), 1.06 (br t, 2H), 1.21 (s, 18H), 3.97 (d, J = 10.0 Hz, 2H), 4.23 (s, 2H), 5.0 (br s, 2H), 5.62 (m, 2H), 8.01 (s, 1H), 8.68 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 12.3 (2C), 26.7 (6C), 38.6 (2C), 46.0, 62.1 (d, J = 170 Hz), 64.1 (d, J = 15.0 Hz), 81.6 (d, J = 6.0 Hz, 2C), 127.6, 143.0, 149.4, 153.4, 158.9, 176.6 (2C). HRMS (MH+): 528.2223 calcd for C10H14N5O4P, found 528.2233. Anal. (C22H34N5O8P) C, H, N.
PATENT
Besifovir dipivoxil’s product PAT, WO2057288
https://encrypted.google.com/patents/WO2002057288A1?cl=en
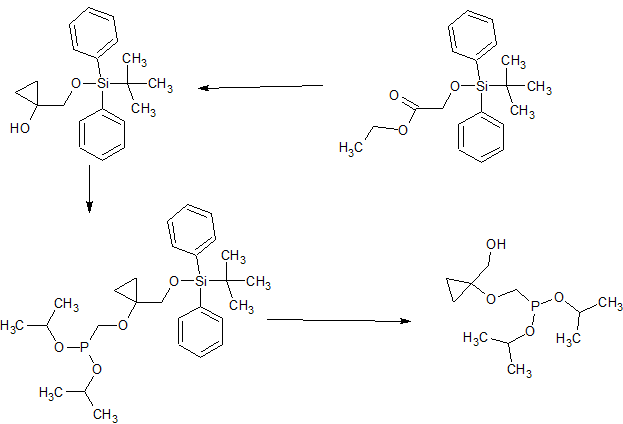
(a) CH3CH2MgBr, Ti(Oi-Pr)4 (0.25 equiv), THF, 0 °C to 25 °C, 10 h;
(b) BrCH2P(O)(Oi-Pr)2, LiOt-Bu, LiI (cat.), DMF, THF, 60 °C, 4 h;
(c) NH4F, MeOH, reflux, 10 h;
CONTD……….
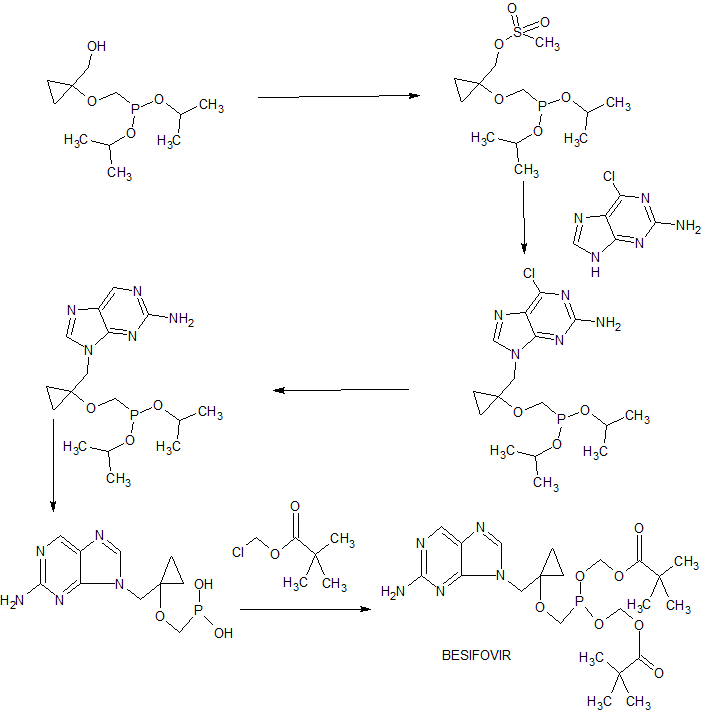
(d) MsCl, TEA, MDC, 0 °C to 25 °C; (e) 6-chloroguanine, NaH, DMF, 80 °C, 4 h; (f) H2, 5% Pd on C, THF, 1 atm, 18 h; (g) TMSBr, MDC, reflux, 18 h; (h) 2 N HCl, reflux, 6 h; (i) chloromethyl pivalate, TEA, 1-methyl-2-pyrrolidinone, 25 °C, 48 h.
WO 2002057288
PATENT
WO-2018016795
Novel crystalline polymorphic forms of 3-[({1-[(2-amino-9H-furyn-9-yl) methyl] cyclopropyl}oxy) methyl]-8,8-dimethyl-3,7-dioxo-2,4,6-trioxa-3λ5-phosphanon-1-yl-pivalate orotate (Besifovir dipivoxil), a process for its preparation, and composition comprising the salt for treating viral infections are claimed.
References
- Jump up^ WHO International Working Group for Drug Statistics Methodology (August 27, 2008). “ATC/DDD Classification (FINAL): New ATC 5th level codes”. WHO Collaborating Centre for Drug Statistics Methodology. Archived from the original on 2008-05-06. Retrieved 2008-09-05.
- Jump up^ Lin CL, Yang HC, Kao JH (4 January 2016). “Hepatitis B virus: new therapeutic perspectives”. Liver Int. John Wiley & Sons Ltd. 36(Supplement S1): 85–92. doi:10.1111/liv.13003. PMID 26725903.
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C10H14N5O4P |
| Molar mass | 299.223022 g/mol |
| 3D model (JSmol) | |
////////////Besifovir, бесифовир , بيسيفوفير , 贝西福韦 , ANA-380, AN-380, ANA 380, LB-80380, LB 80380, LB80380, PMCDG dipivoxil
C1CC1(CN2C=NC3=CN=C(N=C32)N)OCP(=O)(O)O
NC1=NC=C2N=CN(CC3(OCP(OCOC(C(C)(C)C)=O)OCOC(C(C)(C)C)=O)CC3)C2=N1