Cafestol, a Bioactive Substance in Coffee, Has Antidiabetic Properties in KKAy Mice https://lnkd.in/e7BxMkg
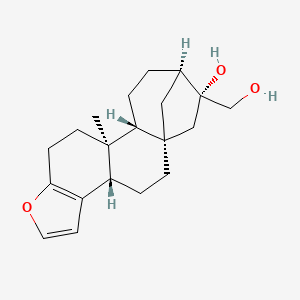
Cafesterol
- Molecular FormulaC20H28O3
- Average mass316.435 Da
-
(1S,4S,12S,13R,16S,
17R)-17-(Hydroxymet hyl)-12-methyl-8-ox apentacyclo[14.2.1. 01,13 .04,12 .05,9 ]nonadeca-5(9),6-di en-17-ol (1S,4S,12S,13R,16S,17R)-17-(Hydroxymet hyl)-12-methyl-8-ox apentacyclo[14.2.1. 01,13 .04,12 .05,9 ]nonadeca-5(9),6-di en-17-ol (1S,4S,12S,13R,16S,17R)-17-(Hydroxymét hyl)-12-méthyl-8-ox apentacyclo[14.2.1. 01,13 .04,12 .05,9 ]nonadéca-5(9),6-di én-17-ol (3bS,5aS,7R,10aR,10bS)-7-(hydroxymethy l)-10b-methyl-3b,4, 5,6,7,8,9,10,10a,10 b,11,12-dodecahydro -5a,8-methanocycloh epta[5,6]naphtho[2, 1-b]furan-7-ol [3bS-(3ba,5ab,7b,8b-10aa,10bb)]-3b,4,5 ,6,7,8,9,10,10a,10b ,11,12-Dodecahydro- 7-hydroxy-10b-methy l-5a,8-methano-5aH- cyclohepta[5,6]-nap htho[2,1-b]furan-7- methanol Cafesterol469-83-0 [RN]
Scientists identify health benefits of cafestol in coffee
Scientists have identified two compounds in coffee – cafestol and caffeic acid – that could someday lead to the development of new medications to better prevent and treat type 2 diabetes…

Drinking three to four cups of coffee per day has been shown to decrease the risk of developing type 2 diabetes.
Now, scientists report they have identified two compounds that contribute to this health benefit. Researchers say that this knowledge could someday help them develop new medications to better prevent and treat the disease.
Patients with type 2 diabetes become resistant to insulin, a hormone that helps turn glucose from food into energy. To overcome this resistance, the pancreas makes more insulin, but eventually, it just can’t make enough. High blood glucose levels can cause health problems, such as blindness and nerve damage.
Coffee’s cafestol has dual benefits
Several genetic and life style risk factors have been linked to the development of type 2 diabetes, but drinking coffee has been shown to help prevent its onset. Caffeine was thought to be responsible, but studies have shown it has only a short-term effect on glucose and insulin, and decaffeinated coffee has the same effect as the regular version of the drink. To investigate which of coffee’s many bioactive components are responsible for diabetes prevention, Søren Gregersen and colleagues tested the effects of different coffee substances in rat cell lines.
The researchers investigated different coffee compounds’ effects on cells in the lab. Cafestol and caffeic acid both increased insulin secretion when glucose was added. The team also found that cafestol increased glucose uptake in muscle cells, matching the levels of a currently prescribed antidiabetic drug. They say cafestol’s dual benefits make it a good candidate for the prevention and treatment of type 2 diabetes. However, because coffee filters eliminate much of the cafestol in drip coffee, it is likely that other compounds also contribute to these health benefits.

 |
|
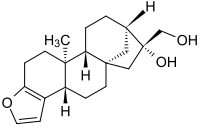
| Names | |
|---|---|
| IUPAC name
(3bS,5aS,7R,8R,10aR,10bS)-3b,4,5,6,7,8,9,10,10a,10b,11,12-Dodecahydro-7-hydroxy-10b-methyl-5a,8-methano-5aH-cyclohepta[5,6]naphtho[2,1-b]furan-7-methanol
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
| Properties | |
| C20H28O3 | |
| Molar mass | 316.44 g·mol−1 |
| Melting point | 158 to 162 °C (316 to 324 °F; 431 to 435 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
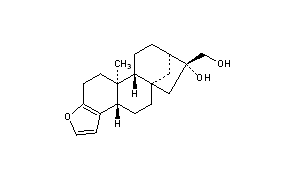 |
| Structural Formula Vector Image |
|
Title: Cafestol
CAS Registry Number: 469-83-0
CAS Name: [3bS-(3ba,5ab,7b,8b,10aa,10bb)]-3b,4,5,6,7,8,9,10,10a,10b,11,12-Dodecahydro-7-hydroxy-10b-methyl-5a,8-methano-5aH-cyclohepta[5,6]naphtho[2,1-b]furan-7-methanol
Additional Names: cafesterol
Molecular Formula: C20H28O3
Molecular Weight: 316.43
Percent Composition: C 75.91%, H 8.92%, O 15.17%
Literature References: Diterpenoid constituent of coffee. Isoln from green coffee oil: Slotta, Neisser, Ber. 71, 1991, 2342 (1938); C. Djerassi et al., J. Org. Chem. 18, 1449 (1953). Prepn and purification: R. Bertholet, US 4692534 (1987 to Nestec). Structure: C. Djerassi et al., J. Am. Chem. Soc. 81, 2386 (1959); R. A. Finnegan, C. Djerassi, ibid. 82, 4342 (1960). Stereochemical studies: R. A. Finnegan, J. Org. Chem. 26, 3057 (1961); A. I. Scott et al., J. Am. Chem. Soc. 84, 3197 (1962); A. I. Scott et al., Tetrahedron20, 1339 (1964). Stereospecific total synthesis of (±)-form: E. J. Corey et al., J. Am. Chem. Soc. 109, 4717 (1987).
Properties: Crystals from hexane, mp 158°-l60°. [a]D -101°. uv max: 222 nm (log e 3.78).
Melting point: mp 158°-l60°
Optical Rotation: [a]D -101°
Absorption maximum: uv max: 222 nm (log e 3.78)
Derivative Type: Acetate
Molecular Formula: C22H30O4
Molecular Weight: 358.47
Percent Composition: C 73.71%, H 8.44%, O 17.85%
Properties: Needles from petr ether, mp 167-168°. [a]D -89°. uv max: 222 nm (log e 3.80).
Melting point: mp 167-168°
Optical Rotation: [a]D -89°
Absorption maximum: uv max: 222 nm (log e 3.80)
Derivative Type: Tetrahydrocafestol
Molecular Formula: C20H32O3
Molecular Weight: 320.47
Percent Composition: C 74.96%, H 10.06%, O 14.98%
Properties: Crystals from dil methanol, mp 154.5-157°.
Melting point: mp 154.5-157°
|
//////////Cafesterol
CC12CCC3=C(C1CCC45C2CCC(C4)C(C5)(CO)O)C=CO3