Centhaquine
PMZ-2010
CAS 57961-90-7
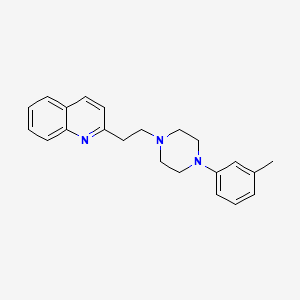
2-[2-[4-(3-methylphenyl)piperazin-1-yl]ethyl]quinoline
INDIA 2020, 14.05.2020, Centhaquine citrate bulk and Centhaquine citrate injection 1.0mg/vial, Add on resuscitative agent for hypovolemic shock
- OriginatorMidwestern University; Pharmazz
- DeveloperPharmazz
- ClassAnalgesics; Antihaemorrhagics; Antihypertensives; Cardiovascular therapies; Piperazines; Quinolines; Small molecules
- Mechanism of ActionAlpha 1 adrenergic receptor antagonists; Alpha 2 adrenergic receptor agonists
- RegisteredHaemorrhagic shock
- Phase IHeart arrest; Postoperative pain
- 20 Jul 2020Pharmazz plans to launch centhaquin for Haemorrhagic shock (Adjuvant therapy) in India by the middle of September 2020
- 20 Jul 2020Efficacy data from a phase III trial in Haemorrhagic shock released by Pharmazz
- 02 Jun 2020Centhaquine is still in phase I trials for Postoperative pain in USA (Pharmazz pipeline, June 2020)
PATENT
https://patents.google.com/patent/WO2014035446A1/en
Shock due to severe hemorrhage accounts for a large proportion of posttraumatic deaths, particularly during early stages of injury (Wu, Dai et al. 2009). A majority of deaths due to hemorrhage occur within the first six hours after trauma (Shackford, Mackersie et al. 1993), but many of these deaths can be prevented (Acosta, Yang et al. 1998).
[0003] Shock is accompanied by circulatory failure which is the primary cause of mortality and morbidity. Presently, the recommended fluid therapy uses large volumes of Lactated Ringer’s solution (LR), which is effective in restoring hemodynamic parameters, but presents logistic and physiologic limitations (Vincenzi, Cepeda et al. 2009). For example, resuscitation using a large volume of crystalloids, like LR, has been associated with secondary abdominal compartment syndrome, pulmonary edema, cardiac dysfunction, and paralytic ileus (Balogh, McKinley et al. 2003). Therefore, a need exists in the art for a resuscitation agent that improves survival time, and can be used with a small volume of resuscitation fluid, for resuscitation in hypovolemic shock.
[0004] Centhaquin (2-[2-(4-(3-methyphenyl)-l-piperazinyl) ethyl-quinoline) is a centrally acting antihypertensive drug. The structure of centhaquin was determined (Bajpai et al., 2000) and the conformation of centhaquin was confirmed by X-ray diffraction (Carpy and Saxena, 1991).
Structure of centhaquin (2-[2-(4-(3-methyphenyl)- 1 -piperazinyl) ethyl] -quinoline) (as free base)
[0005] Centhaquin is an active cardiovascular agent that produces a positive inotropic effect and increases ventricular contractions of isolated perfused rabbit heart (Bhatnagar, Pande et al. 1985). Centhaquin does not affect spontaneous contractions of the guinea pig right auricle, but significantly potentiates positive inotropic effect of norepinephrine (NE) (Srimal, Mason et al. 1990). The direct or indirect positive inotropic effect of centhaquin can lead to an increase in cardiac output (CO). Centhaquin produces a decrease in mean arterial pressure (MAP) and heart rate (HR) in anesthetized rats and conscious freely moving cats and rats (Srimal, Gulati et al. 1990) due to its central sympatholytic activity (Murti, Bhandari et al. 1989; Srimal, Gulati et al. 1990; Gulati, Hussain et al. 1991). When administered locally into a dog femoral artery, centhaquin (10 and 20 μg) increased blood flow, which was similar to that observed with acetylcholine and papaverine. However, the vasodilator effect of centhaquin could not be blocked by atropine or dibenamine (Srimal, Mason et al. 1990). The direct vasodilator or central sympatholytic effect of centhaquin is likely to decrease systemic vascular resistance (SVR).
[0006] It was found that centhaquin enhances the resuscitative effect of hypertonic saline (HS) (Gulati, Lavhale et al. 2012). Centhaquin significantly decreased blood lactate and increases MAP, stroke volume, and CO compared to hypertonic saline alone. It is theorized, but not relied upon, that the cardiovascular actions of hypertonic saline and centhaquin are mediated through the ventrolateral medulla in the brain (Gulati, Hussain et al. 1991 ; Cavun and Millington 2001) and centhaquin may be augmenting the effect of hypertonic saline.
[0007] A large volume of LR (i.e., about three times the volume of blood loss) is the most commonly used resuscitation fluid therapy (Chappell, Jacob et al. 2008), in part because LR does not exhibit the centrally mediated cardiovascular effects of hypertonic saline. Large volume resuscitation has been used by emergency medical personnel and surgeons to reverse hemorrhagic shock and to restore end-organ perfusion and tissue oxygenation. However, there has been a vigorous debate with respect to the optimal methods of resuscitation (Santry
ased on the molecular weight of centhaquin (free base) (MW-332) and centhaquin citrate (MW-523), for identical doses of centhaquin (as free base) and centhaquin citrate, centhaquin citrate provides only 63.5% of centhaquin free base compared to the dose of centhaquin free base, e.g., a 0.05 mg dose of centhaquin citrate contains a 0.0318 mg of centhaquin (as free base). Similarly, a dose of centhaquin citrate dihydrate (MW-559) provides 59.4% centhaquin (free base) of the same dose as centhaquin (as free base), i.e., a 0.0005 mg dose of centhaquin citrate dihydrate contains 0.030 mg of centhaquin (as free base). Surprisingly, and as demonstrated below, at the same mg/kg dose centhaquin citrate and centhaquin citrate dihydrate provides greater cardiovascular effects than centhaquin free base.
Synthesis of Centhaquin
[0061] The synthesis of centhaquin was reported by Murthi and coworkers (Murthi et al U.S. Patent No. 3,983,121 ; Murti, Bhandari et al. 1989). In one procedure, reactants 1 and 2 were stirred at reflux for 15 hours. The resulting product was purified by evaporating the solvents to obtain an oil, which was heated in vacuo (100°C, 1 mm Hg). The remaining residue was recrystallized from ether-petroleum ether to obtain the final centhaquin product 3. The melting point reported for centhaquin was 76-77°C. In a subsequent publication (Murti, Bhandari et al. 1989), the reaction mixture was concentrated following 24 hours of reflux, diluted with water, and basified with aqueous NaOH. The basic mixture was extracted with ethyl acetate, and the ethyl acetate extracts were dried over anhydrous sodium sulfate and evaporated in vacuo to give centhaquin which was crystallized from hexane. The melting point of centhaquin (free base) obtained in this procedure was 82°C. The product obtained using either purification method is light tan in color, which is indicative of small amounts of impurities that were not completely removed using previously reported purification methods.
[0062] In accordance with the present invention, an improved purification method was found. According to the improved method, reactants 1 and 2 were stirred at reflux for 24 hours. The solvents were evaporated in vacuo and the resulting mixture was diluted with water and basified (10% NaOH). The basic mixture was extracted with ethyl acetate and the combined ethyl acetate extracts are dried over anhydrous sodium sulfate and evaporated in vacuo to obtain a residue, which was further purified with column chromatography (Si02, ethyl acetate). The resulting product can be decolorized using activated charcoal or directly crystallized from hot hexane to yield pure centhaquin. The resulting product is an off-white crystalline solid having a melting point of 94-95°C (free base). The product was
characterized using proton NMR, mass spectral, and elemental analysis and indicated high purity and superior quality.
[0063] Synthesis and characterization of centhaquin (free base): A mixture of 2- vinylquinoline (1) (5.0 g, 32.2 mmol, 98.5%) and 1 -(3-methylphenyl)piperazine (2) (5.68 g, 32.2 mmol, 99.0%) in absolute ethyl alcohol (150 ml) and glacial acetic acid (3.5 ml) was stirred at reflux for 24 hours in a round bottom flask. The reaction mixture was concentrated in vacuo, diluted with water (150 ml) and treated with 10% aqueous NaOH (150 ml). The residue was extracted with ethyl acetate (4 x 125 ml), dried with anhydrous Na2S04, and concentrated under reduced pressure to yield a crude product which was purified by column chromatography using silica gel (100-200 mesh) with ethyl acetate as an eluent. The resulting compound was recrystallized from hot hexane and filtered, to yield centhaquin as an off- white crystalline solid (7.75 g, 23.4 mmol, 73% yield); mp. 94-95°C; i? 0.30 (100% ethyl acetate); 1H NMR (300 MHz, CDC13): δ 8.07 (t, J= 7.5 Hz, 2 H), 7.78 (d, J= 7.8 Hz, 1 H), 7.70 (t, J= 7.8 Hz, 1 H), 7.50 (t, J= 7.5 Hz, 1 H), 7.36 (d, J= 8.4 Hz, 1 H), 7.16 (t, J = 7.5 Hz, 1 H), 6.77 – 6.74 (m, 2 H), 6.69 (d, J= 7.2 Hz, 1 H), 3.26- 3.21 (m, 6 H), 2.97 – 2.92 (m, 2 H), 2.76 – 2.73 (m, 4 H), 2.32 (s, 3 H); HRMS (ESI) m/z 332.2121 [M+l]+ (calcd for C22H26N3 332.2122); Anal. (C22H25N3) C, H, N.
[0064] Preparation of centhaquin citrate: Centhaquin (free base) (5.62 g, 16.98 mmol) was treated with citric acid (3.26 g, 16.98 mmol) in a suitable solvent and converted to the citrate salt obtained as an off-white solid (7.96 g, 15.2 mmol, 90%); m.p. 94-96°C ; Anal.
10065] Figs. 1(a) and 1(b) are high resolution mass spectral analyses of centhaquin free base (Fig 1(a)) and centhaquin citrate (Fig. 1(b)). Compound samples were analyzed following ionization using electrospray ionization (ESI).
[0066J For centhaquin free base in Fig 1(a), a base peak [M+l]+ was observed at m z 332.2141 (theory: 332.2121) consistent with the elemental composition of protonated centhaquin (C22H26N3).
[0067] For centhaquin citrate in Fig 1(b), the mass spectrum was identical to the mass spectrum obtained for the free base. An [M+l]+base peak was observed at m z 332.2141 (theory: 332.2121), which corresponds to the elemental composition of protonated centhaquin (C22H26N3). This result is typical of salts of organic bases to yield the [M+l]+ of the free base as observed here with centhaquin citrate.
[0068] Mass spectrometry is one of the most sensitive analytical methods, and examination of the mass spectra of Fig. 1 indicate that the samples are devoid of any extraneous peaks and are of homogeneous purity (>99.5).
PATENT
https://patents.google.com/patent/WO2014035446A1/en
////////////Centhaquine, PMZ-2010, PMZ 2010, INDIA 2020, 2020 APPROVALS