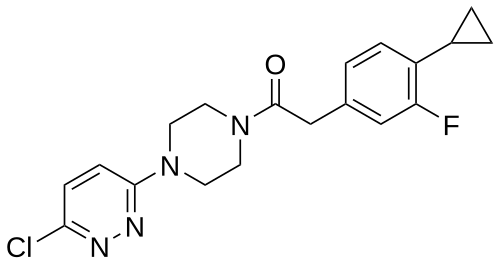
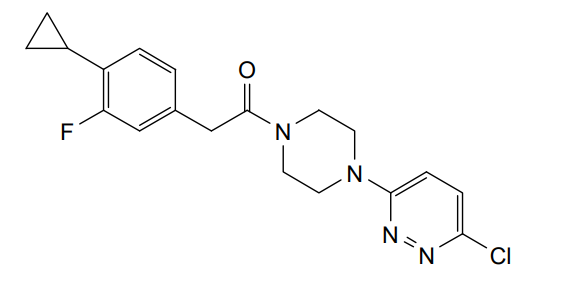
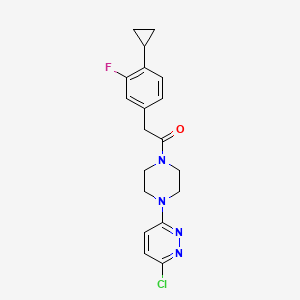
Claziprotamide
CAS 2361124-03-8, BBP 671
MF C19H20ClFN4O MW374.8 g/mol
1-[4-(6-chloropyridazin-3-yl)piperazin-1-yl]-2-(4-cyclopropyl-3-fluorophenyl)ethan-1-one
1-[4-(6-chloropyridazin-3-yl)piperazin-1-yl]-2-(4-cyclopropyl-3-fluorophenyl)ethan-1-one
pantothenate kinases 1 and 3 (PanK1 and PanK3) positive allosteric modulator
Claziprotamide is an investigational new drug that is being evaluated for the treatment of rare metabolic disorders such as pantothenate kinase-associated neurodegeneration (PKAN) and neurodegeneration with brain iron accumulation (NBIA). It acts as a positive allosteric modulator (PAM) of pantothenate kinases 1 and 3 (PANK1 and PANK2) which are critical for coenzyme A biosynthesis and cellular metabolism.[1][2]
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US319558593&_cid=P22-MG32VL-67777-1
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019133635&_cid=P22-MG32PO-63930-1
SCHEME 4B.
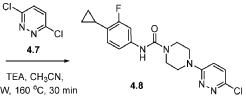
[00184] In one aspect, compounds of type 4.8, and similar compounds, can be prepared according to reaction Scheme 4B above. Thus, compounds of type 4.6 can be prepared by a urea bond formation reaction between an appropriate amine, e.g., 4.2 as shown above, and an appropriate isocyanate, e.g., 4.5 as shown above. Appropriate amines and appropriate isocyanates are commercially available or prepared by methods known to one skilled in the art. The nucleophilic substitution is carried out in the presence of an appropriate solvent, e.g., diethyl ether, for an appropriate period of time, e.g., 3 hours. The nucleophilic substitution is followed by a deprotection reaction. The deprotection reaction is carried out in the presence of an appropriate deprotecting agent, e.g., trifluoroacetic acid, in an appropriate solvent, e.g., dichloromethane, for an appropriate period of time, e.g., 1 hour. Compounds of type 4.8 can be prepared by an arylation reaction of appropriate amine, e.g., 4.6 as shown above, and an appropriate aryl halide, e.g., 4.7 as shown above. Appropriate aryl halides are commercially available or prepared by methods known to one skilled in the art. The arylation reaction is carried out in the presence of an appropriate base, e.g., triethylamine, in an appropriate solvent, e.g., acetonitrile, at an appropriate temperature, e.g, 160 °C, for an appropriate period of time, e.g., 30 minutes using microwave irradiations. As can be appreciated by one skilled in the art, the above reaction provides an example of a generalized approach wherein compounds similar in structure to the specific reactants above (compounds similar to compounds of type 3.6, 4.1, 4.2, and 4.3), can be substituted in the reaction to provide 4-aryl-N-phenylpiperazine-l -carboxamide derivatives similar to Formula 4.4.
PAT
- Methods of treating disorders associated with castorPublication Number: US-2023321092-A1Priority Date: 2017-12-27
- Small molecule modulators of pantothenate kinasesPublication Number: US-11891378-B2Priority Date: 2017-12-27Grant Date: 2024-02-06
- Small molecule modulators of pantothenate kinasesPublication Number: US-2024287039-A1Priority Date: 2017-12-27
- Small molecule modulator of pantothenate kinasePublication Number: KR-102728619-B1Priority Date: 2017-12-27Grant Date: 2024-11-08
- Small molecule modulators of pantothenate kinasesPublication Number: US-2021061788-A1Priority Date: 2017-12-27
- Methods of treating disorders associated with castorPublication Number: US-11547709-B2Priority Date: 2017-12-27Grant Date: 2023-01-10
- Small molecule modulators of pantothenate kinasesPublication Number: AU-2018395222-B2Priority Date: 2017-12-27Grant Date: 2023-06-08
- Small molecule modulators of pantothenate kinasePublication Number: CN-111818922-BPriority Date: 2017-12-27Grant Date: 2023-06-13
- Small molecule modulators of pantothenate kinasePublication Number: JP-7352565-B2Priority Date: 2017-12-27Grant Date: 2023-09-28
- Small molecule modulators of pantothenate kinasesPublication Number: EP-3731844-A1Priority Date: 2017-12-27
- Small molecule modulators of pantothenate kinasePublication Number: KR-20200130242-APriority Date: 2017-12-27
- MODULATORS OF SMALL MOLECULES OF PANTOTENATE KINASESPublication Number: BR-112020012875-A2Priority Date: 2017-12-27
- Small molecule modulator of pantothenate kinasePublication Number: JP-2021508739-APriority Date: 2017-12-27
- Methods of treating disorders associated with castorPublication Number: US-2021023081-A1Priority Date: 2017-12-27
- Treatment of organic acidemias or pantothenate kinase associated neurodegeneration with modulators of pantothenate kinasesPublication Number: WO-2023230560-A1Priority Date: 2022-05-26
- Methods of treating disorders associated with castorPublication Number: WO-2022133034-A1Priority Date: 2020-12-16
- Small molecule modulators of pantothenate kinasesPublication Number: WO-2019133635-A1Priority Date: 2017-12-27
- Small molecule modulators of pantothenate kinasesPublication Number: AU-2018395222-A1Priority Date: 2017-12-27
- Small molecule modulators of pantothenate kinasePublication Number: CN-111818922-APriority Date: 2017-12-27



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | BBP-671 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2361124-03-8 |
| PubChem CID | 142616838 |
| ChemSpider | 129431674 |
| UNII | 74N47PKZ3K |
| PDB ligand | Y92 (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C19H20ClFN4O |
| Molar mass | 374.84 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- Tangallapally R, Subramanian C, Yun MK, Edwards A, Sharma LK, Yang L, et al. (August 2024). “Development of Brain Penetrant Pyridazine Pantothenate Kinase Activators”. Journal of Medicinal Chemistry. 67 (16): 14432–14442. doi:10.1021/acs.jmedchem.4c01211. PMC 11345825. PMID 39136313.
- “Claziprotamide”. PatSnap.
/////////Claziprotamide, BBP 671, ORPHAN DRUG














