
Clenbuterol
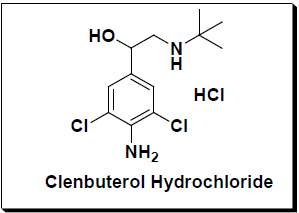
- Clenbuterol hydrochloride, NAB-365, Siropent
Clenbuterol, marketed as Dilaterol, Spiropent, Ventipulmin,[1] is a sympathomimetic amine used by sufferers of breathing disorders as a decongestant and bronchodilator. People with chronic breathing disorders such as asthma use this as a bronchodilator to make breathing easier. It is most commonly available as the hydrochloride salt, clenbuterol hydrochloride.[2]

Effects and dosage
Clenbuterol is a β2 agonist with some structural and pharmacological similarities to epinephrine and salbutamol, but its effects are more potent and longer-lasting as a stimulant and thermogenic drug. It causes an increase in aerobic capacity, central nervous system stimulation, blood pressure, and oxygen transportation. It increases the rate at which body fat is metabolized while increasing the body’s basal metabolic rate (BMR). It is commonly used for smooth muscle-relaxant properties as a bronchodilator and tocolytic.
Clenbuterol is also prescribed for treatment of horses, but equine use is usually the liquid form.
Human use
Clenbuterol is approved for use in some countries, free or via prescription, as a bronchodilator for asthma patients.[3]

Legal status
Clenbuterol is not an ingredient of any therapeutic drug approved by the US Food and Drug Administration[3] and is now banned forIOC-tested athletes.[4] In the US, administration of clenbuterol to any animal that could be used as food for human consumption is banned by the FDA.[5][6]
Clenbuterol is a therapeutic drug for asthma and COPD, approved for human use in some countries in Europe (Bulgaria and Russia) and Asia (China).

Weight-loss drug
Although often used by bodybuilders during their “cutting” cycles,[citation needed] the drug has been more recently known to the mainstream, particularly through publicized stories of use by celebrities such as Victoria Beckham,[4] Britney Spears, and Lindsay Lohan, [7] for its off-label use as a weight-loss drug similar to usage of other sympathomimetic amines such as ephedrine, despite the lack of sufficient clinical testing either supporting or negating such use.



By bromination of 4-amino-3,5-dichloroacetophenone (I) with Br2 in CHCl3 to give 4-amino-3,5-dichloro-alpha-bromoacetophenone (II), m.p. 140-5 C, which is condensed with tert-butylamine (III) in CHCl3 to 4-amino-3,5-dichloro-alpha-tertbutylaminoacetophenone hydrochloride (IV), m.p. 252-7 C; this product is finally reduced with NaBH4 in methanol.
Synthesen von neuen Amino-Halogen-substituierten Phenyl-aminothanolen. Arzneim-Forsch Drug Res 1972, 22, 5, 861-869
CLIP
Synthesis and Characterization of Bromoclenbuterol
Ravi Kumar Kannasani*, Srinivasa Reddy Battula, Suresh Babu Sannithi, Sreenu Mula and Venkata Babu VV
R&D Division, RA Chem Pharma Limited, API, Hyderabad, Telangana, India
- *Corresponding Author:
- Ravi Kumar Kannasani
R&D Division, RA Chem Pharma Limited
API, Prasanth Nagar, Hyderabad, Telangana, India
Tel: +919000443184
E-mail: kannasani.ravi@rachempharma.com
Citation: Kannasani RK, Battula SR, Sannithi SB, Mula S, Babu VVV (2016) Synthesis and Characterization of Bromoclenbuterol. Med Chem (Los Angeles) 6:546-549. doi:10.4172/2161-0444.1000397
Clenbuterol, it is most commonly available as the hydrochloride salt, clenbuterol hydrochloride. Clenbuterol, marketed as Dilaterol, Spiropent, Ventipulmin, and also generically as clenbuterol, is a sympathomimetic amine used for breathing disorders as a decongestant and bronchodilator. People with chronic breathing disorders such as asthma use this as a bronchodilator to make breathing easier. Clenbuterol is a β2 agonist with some structural and pharmacological similarities to epinephrine and salbutamol, but its effects are more potent and longerlasting as a stimulant and thermogenic drug. It causes an increase in aerobic capacity, central nervous system stimulation, blood pressure, and oxygen transportation. It increases the rate at which body fat is metabolized while increasing the body’s BMR. It is commonly used for smooth muscle-relaxant properties as a bronchodilator and tocolytic. Clenbuterol is also prescribed for treatment of horses, but equine use is usually the liquid form
Clenbuterol Hydrochloride was first synthesized at Thomae; a Boehringer Ingelheim research facility in Biberach, Germany, in 1967. The synthesis of Clenbuterol Hydrochloride was patented in the United States in 1970. After comprehensive clinical trials, Clenbuterol Hydrochloride was approved for the treatment of reversible airway obstruction in Germany in 1976 and later as a veterinary pharmaceutical for the treatment of bronchiolytic disorders in Germany in 1980. Boehringer Ingelheim markets Clenbuterol Hydrochloride as Spirospent for Human Pharmaceuticals and as Ventipulmin for Veterinary Pharmaceuticals. Clenbuterol Hydrochloride is not approved by the Federal Drug Administration for human use in the United States.
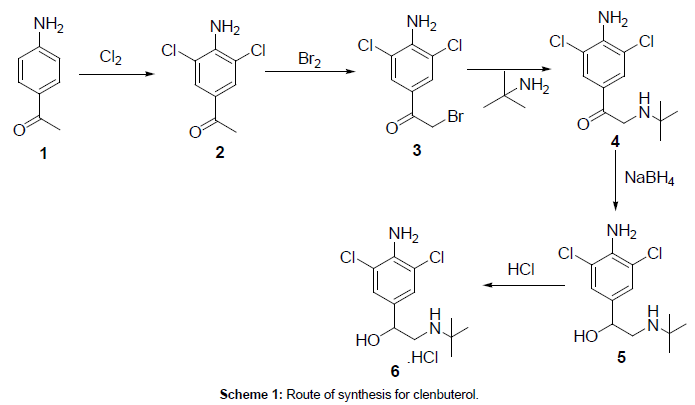
As per the available literature [4–7], clenbuterol hydrochloride was synthesized from 4-amino acetophenone (Scheme 1). Initially 4-amino acetophenone (1) was reacted with chlorine to afford 4-amino-3,5- dichloro acetopheneone (2) which was further reacted bromine to give 1-(4-amino-3,5-dichlorophenyl)-2-bromoethanone (3). The obtained bromo compound was reacted tertiary butyl amine to afford 2-(tertbutylamino)- 1-(4-amino-3,5-dichlorophenyl)ethanone (4), which was further reduced with sodium borohydride to give clenbuterol base (5) and converted in to hydrochloride salt by using alcoholic HCl to get clenbuterol hydrochloride (6).
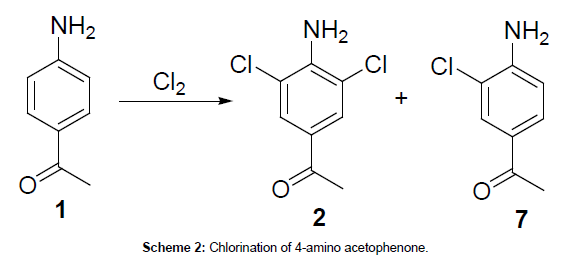
In the synthesis of clenbuterol hydrochloride, first step was a double chlorination of 4-aminoacetophenone (1) through an electrophillic aromatic substitution reaction to yield 4-amino-3,5- dichloroacetophenone (2). Due to the ortho/para directing, amino group and the meta directing, electron withdrawing, acetyl group, chlorination of 4-aminoacetophenone occurs primarily at the 3 and 5 positions over the 2 and 6 positions. Therefore, under chlorination would produce only the mono chlorinated impurity, 4-amino-3- chloroacetophenone. Under these conditions, over chlorination does not result in the addition of chlorine to the 2 and 6 positions because the amino and acetyl groups do not direct that addition. Even though chlorides are ortho/para directing and direct to the 2 and 6 position, chlorides are also deactivating. After close observation on this chlorination reaction, it was noted that the formed mono chlorinated impurity (Scheme 2) (4-amino-3-chloro acetophenone) caused the formation of process related impurity (bromoclenbuterol) in clenbuerol synthesis.
References for above
- International Conference on Harmonization (ICH) Guidelines (2002) Q3A (R) Impurities in New Drug Substances.
- ICH (2006) Impurities in New Drug Products. Q3B (R1).
- Srinivasulu R, Ravi Kumar K, Nageswararao K (2016) Synthesis of 3,4-dihydropyrimidin-2(1H)-ones using camphor sulfonic acid as a catalyst under solvent-free conditions. Derpharma chemica 8: 375-379.
- Antuna AZ, Ivan L, Pablo RG, Julio R, Jose, et al. (2011) V. Tetrahedron 67: 5577-5581.
- Ehrhardt JD (1990) Synthesis of nonadeutero-clenbuterol. Journal of labeled compounds and radiopharmaceuticals 28: 725-729.
- Drabb TW (1990) Method for the preparation of 4′-(substituted)-amino-2-(substituted) Amino-3’,5′-dichloroacetophenone and salts thereof Trenton. NJ Patent: US4906781A.
- Bentley TJ (1984) Method for the preparation of 1-(4′-amino-3′,5′-dichlorophenyl)-2-alkyl(or dialkyl)aminoethanols. US4461914 A.

References
- Jump up^ Medicine, Center for Veterinary. “FOIA Drug Summaries – NADA 140-973 VENTIPULMIN® SYRUP – original approval”. www.fda.gov. Retrieved 2016-03-10.
- Jump up^ “874. Clenbuterol (WHO Food Additives Series 38)”. www.inchem.org. Retrieved2016-03-10.
- ^ Jump up to:a b “Clenbuterol”. Daily Mail. 2009-10-01. Retrieved 2010-04-07
- ^ Jump up to:a b Guest, Katy (2007-04-10). “Clenbuterol: The new weight-loss wonder drug gripping Planet Zero”. The Independent. London. Retrieved 2007-04-10.
- Jump up^ FDA’s Prohibited Drug List, Food Animal Residue Avoidance & Depletion Program
- Jump up^ “Animal Drugs @ FDA”. www.accessdata.fda.gov. Retrieved 2016-03-10.
- Jump up^ “Clenbuterol Weight Loss Hollywood Secret”. PRBuzz. London. 2012-05-17. Retrieved2012-04-10.
- Jump up^ Philip Hersh – Series on Athletics in the GDR
- Jump up^ “Krabbe receives IAAF settlement”. BBC News. 2002-04-30.
- Jump up^ [1][dead link]
- Jump up^ Guillermo Mota of San Francisco Giants gets 100-game drug suspension
- Jump up^ Dittmeier, Bobbie (May 7, 2012). “Mota suspended 100 games for positive test”.MLB.com. Major League Baseball. Retrieved May 7, 2012.
- Jump up^ Snyder, Whitney (2010-09-30). “Alberto Contador Tests Positive For Banned Substance”. Huffington Post.
- Jump up^ Radioshack suspends Li after doping positive
- Jump up^ “Three Minor League players suspended”. MLB.com. September 30, 2010.
- Jump up^ Macur, Juliet (29 September 2010). “With Positive Test, Contador May Lose Tour Title”.The New York Times. Retrieved 29 September 2010.
- Jump up^ CAS Sanction Contador with two year ban in clenbuterol case, cyclingnews.com, 6 February 2012
- Jump up^ http://www.tas-cas.org/d2wfiles/document/5649/5048/0/Media20Release20_English_2012.02.06.pdf
- Jump up^ “Michael Rogers cleared to race as UCI accepts contaminated meat claim”.theguardian.com. 23 April 2014. Retrieved 24 April 2014.
- Jump up^ “FIFA alarmed by use of food supplements”. September 5, 2012.
- Jump up^ Clenbuterol found in most players at Under-17 World Cup – ESPN
- Jump up^ Boxer Erik Morales banned for two years for failed drug test – BBC Sport
- Jump up^ Leafs’ Ashton suspended 20 games for violating PED policy – Article – TSN
- Jump up^ swim swam.com
- Jump up^ Antidopingový výbor ČR
- Jump up^ http://bigstory.ap.org/article/1c81c75a99cc4bbea4d97cbc90d9e6df/yankees-minor-league-pitcher-cedeno-suspended-72-games
- Jump up^ Collingwood players Lachie Keeffe and Josh Thomas accept two-year bans for clenbuterol positive test – ABC News (Australian Broadcasting Corporation)
- Jump up^ No Cookies | Herald Sun
- Jump up^ “Heavyweight champ ‘Big Daddy’ Browne seeking legal advice over banned substance reports”. ABC News. Retrieved 2016-03-22.
- Jump up^ “Australia’s first world heavyweight champion boxer Lucas Browne fails drug test”. The Sydney Morning Herald. Retrieved 2016-03-22.
- Jump up^ “Raul Mondesi Jr. suspended 50 games for PEDs found in cold medicine”. CBS News. May 10, 2016.
- Jump up^ Francisco Vargas issued temporary license after failed drug test – Ring TV
- Jump up^ “Clenbuterol – SteroidAbuse .com”. www.steroidabuse.com. Retrieved 2016-03-10.
- Jump up^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 325–326.
- Jump up^ “Horse meat investigation. Advice for consumers”. Enforcement and regulation. Food Standards Agency. Retrieved 19 May 2013.
- ^ Jump up to:a b “Clenbuterol”, Food Safety and Inspection Service (FSIS), July 1995, Retrieved 8 April 2015
- Jump up^ China bans production, sale of clenbuterol to improve food safety Retrieved 08/22/2012
- Jump up^ European Commission Retrieved 08/22/2012
- Jump up^ Anti Doping Advisory Notes Retrieved 08/22/2012
- Jump up^ “Pigs fed on bodybuilder steroids cause food poisoning in Shanghai”. AFP. 2006-09-19. Retrieved 2006-09-19.
- Jump up^ “China: 70 ill from tainted pig organs”. CNN. 2009-02-23. Retrieved 2010-04-30.
- Jump up^ Wang Ying (2009-02-23). “70 ill after eating tainted pig organs”. China Daily.
- Jump up^ “China to launch one-year crackdown on contaminated pig feed – xinhuanet.com”.Xinhua. 2011-03-28. Retrieved 2011-03-29.
- Jump up^ Bottemiller, helena (April 26, 2011). “Amid Scandal, China Bans More Food Additives”.Food Safety News. Retrieved August 22, 2012.
- Jump up^ “Import Alert 68-03”. www.accessdata.fda.gov. Retrieved 2016-03-10.
- Jump up^ Planipart Solution for Injection 30 micrograms/ml: Uses, National Office of Animal Health
External links
- Charles F. Kearns; Kenneth H. McKeever; Karyn Malinowski; Maggie B. Struck; Takashi Abe (2001). “Chronic administration of therapeutic levels of clenbuterol acts as a repartitioning agent”. J Appl Physiol. 91 (5): 2064–2070. PMID 11641345.
 |
|
Clenbuterol (top),
and (R)-(−)-clenbuterol (bottom) |
|
| Systematic (IUPAC) name | |
|---|---|
|
(RS)-1-(4-Amino-3,5-dichlorophenyl)-2-(tert-butylamino)ethan-1-ol
|
|
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration |
Oral (tablets, oral solution) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 89–98% (orally) |
| Metabolism | Hepatic (negligible) |
| Biological half-life | 36–48 hours |
| Excretion | Feces and urine |
| Identifiers | |
| CAS Number | 37148-27-9 |
| ATC code | R03AC14 (WHO)R03CC13 (WHO)QG02CA91 (WHO) |
| PubChem | CID 2783 |
| DrugBank | DB01407 |
| ChemSpider | 2681 |
| UNII | XTZ6AXU7KN |
| KEGG | D07713 |
| ChEBI | CHEBI:174690 |
| ChEMBL | CHEMBL49080 |
| Chemical data | |
| Formula | C12H18Cl2N2O |
| Molar mass | 277.19 |
| Chirality | Racemic mixture |
///////////















Hi, I am a bodybuilding on holiday and wont try clenbuterol, i don’t wont buy online i prefere to meet someone to be sure, thanks!