Clobetasol propionate
- Molecular FormulaC25H32ClFO5
- Average mass466.970 Da
CCI 4725, CCI-4725, GR 2/925, GR-2/925,
Active Moieties
| NAME | KIND | UNII | CAS | INCHI KEY |
|---|---|---|---|---|
| Clobetasol | prodrug | ADN79D536H | 25122-41-2 | FCSHDIVRCWTZOX-DVTGEIKXSA-N |
Clobetasol propionate is a corticosteroid used to treat skin conditions such as eczema, contact dermatitis, seborrheic dermatitis, and psoriasis.[2] It is applied to the skin as a cream, ointment, or shampoo.[2][3] Use should be short term and only if other weaker corticosteroids are not effective.[3] Use is not recommended in rosacea or perioral dermatitis.[2]
Common side effects include skin irritation, dry skin, redness, pimples, and telangiectasia.[2] Serious side effects may include adrenal suppression, allergic reactions, cellulitis, and Cushing’s syndrome.[2] Use in pregnancy and breastfeeding is of unclear safety.[4] Clobetasol is believed to work by activating steroid receptors.[2] It is a US Class I (Europe: class IV) corticosteroid, making it one of the strongest available.
Clobetasol propionate was patented in 1968 and came into medical use in 1978.[5] It is available as a generic medication.[3] In 2019, it was the 180th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[6][7]
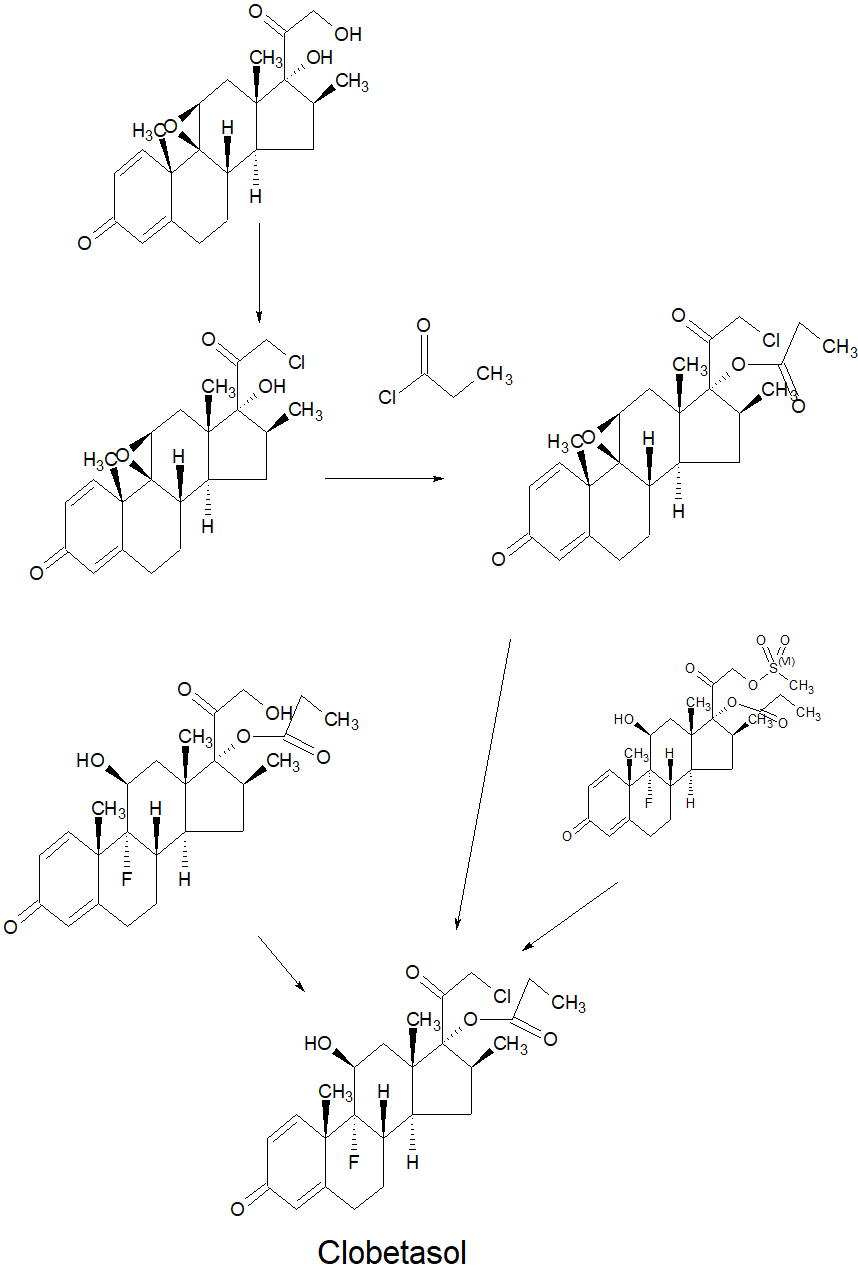
SYNTHESIS OF KEY INTERMEDIATE
SYN
DE 1902340
US 3992422
DE 2613875
EP 72200
WO 2012122452
CN 112110972
PATENT
IN 201821008147
Clobetasol propionate (C25H32ClFO5); CAS Registry No.[25112-46-7]; IUPAC name: 17-(2′- Chloroacetyl)-9-fluoro-l l-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,l 1,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propionate is a potent halogen adrenal corticosteroid of the gluco-corticoid class used to treat various skin disorders including eczema and psoriasis. It is also highly effective for contact dermatitis caused by exposure to poison ivy/oak.In the US 3721687Apatentshow use of methanesulfonyl chloride and Pyridine as base to protect alcohol and at the time of LiCI reaction results with 10-15%ene impurity and less yield.In the methanesulfonyl chloride step used with pyridine as base which is a hazardous.Mesyl compound converted to Clobetasol propionate by using LiCl in Dimethylformamide reaction at IOO-IlO0C forms 10-15% with ene impurity. The synthesis of Clobetasol propionate results in small quantities of the eneimpurity. Clobetasol propionate desired compound to be with impurities which must be minimized. Ene impurity can be reduced to very low levels by reaction itself. However, if used recrystallization reduce ene impurity it is time consuming and very expensive. Further, because recrystallizations have high losses, unacceptably low yields.
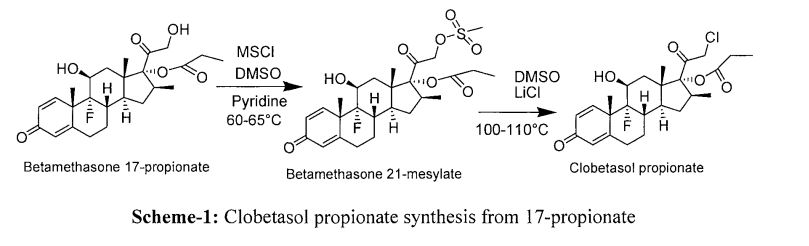
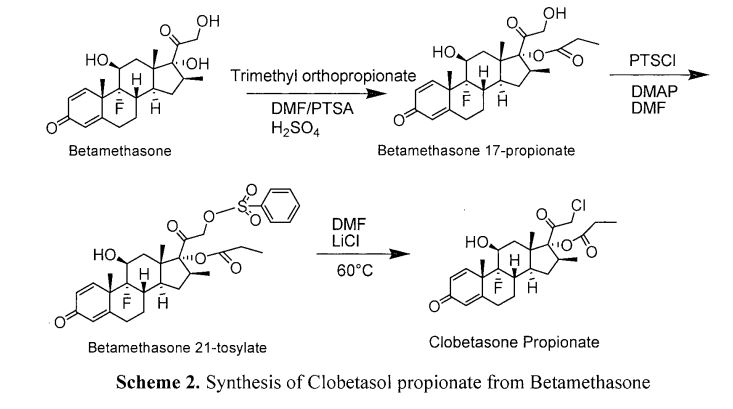
Example I: Betamethasone to betamethasone 17- propionate To a 100 ml 4-neck round bottom flask (RBF) equipped with halfmoon stirrer, thermowelland addition funnel, mounted in a tub bath, was charged betamethasone (5.0g, 0.0127mole), Dimethylformamide (20ml). Cooled the reaction mass to 10-15°C. Slowly added trimethyl ortho propionate (3.42g, 0.0255mole) and p-toluenesulfonic acid (PTSA)(0.30g, 0.00174 mole) to the reaction mass at 10-15°C. Stirred the contents 10-15°C for 4 hr. The reaction was monitored for completion by TLC. Further continued stirring at the same temperature for Ihr till reaction complies by TLC. After reaction completion, added H2SO4UP to pH=1.0-2.0 in to reaction mass.Reaction mass was quenched in Purified water (25ml) at 25-30°C. Cooled reaction mass temperature to 0-5°C. Stirred for I hr and filtered and washed with Purified water (10mlX2). Suck dried under vacuum completely to get cream coloured solid. Dried in tray drier at 50-55°C.Dry weight-5.40g(94.50%); HPLC: 98.5%;mp215-218°C. IR (KBr, on’):3454.90, 3370.99 (-OH); 1719.86, 1659.10 (C=O)iC25H33FO6;
MS 448.52m/z 449.2255 [M+H]; 1HNMR (300MHz, CDCl3S ppm): Spectrum is recorded on Varian, and Tetra Methyl Silane (TMS) as internal standard. 1H-NMR Spectrum shows Aromatic-HK-7.17-7.22(d,lH); Hj-6.37-6.38 (d,lH);Hr6.14 (s,lH); HF-4.04-4.06(s,2H);HE2.23-2.28 (q,2H);Hc-l.39-1.43 (d,3H); HB-1.14 (t,3H);HA-0.96-2.96 (m,20H). 13CMR (300MHz, CDCl3Sppm): 8.692 (CH2-CH3); 16.693; 19.664; 21.353; 23.042; 27.568; 30.387; 36.456; 43.400; 46.547; 47.422; 71.760; 93.547; 124.307; 125.728; 127.903; 129.222; 130.443; 132.472; 145.632; 153.044; 167.424; 175.031 (O-C=O); 185.772 (Cyclic C=O); 196.732 (CH-CO-CH2-OH).
Example 2: Betamethasone 17- propionateto betamethasone 21-tosylate 100ml 4-neck RBF equipped with halfmoon stirrer, thermowell, reflux condenser mountained in water bath, was charged Stage-1 (5.0g, O.Olllmole), Dimethylformamide (20ml). Added 4-Dimethylaminopyridine as base (4.10g, 0.0335mole) and p-toluenesulfonyl chloride (4.24.Og, 0.0222mole)slowly, Stirredfor2-3 hr at 25-30°C. Stirred reaction mass at 25-30°C till reaction complies by TLC.As such reaction mass used insitue for next step. Reaction mass aliquot taken (2ml) and quenched in DM water (20ml), precipited material fdtered and washed with DM water (20ml). Suck dried well. Dried in tray drier at 50-55°C to get dry white solid. Dry weight-0.598g, (89.0%); HPLC: 98.5%; mp-170-175°C (dec). IR (KBr,cm”1):3291.91, 2980.39 (-OH); 1739.15, 1661.99 (C=O); C32H39FO8S; MS 602.71mA 603.2317 [M+H]; 1HNMR (300MHz, CDCl3Sppm): Spectrum is recorded on Varian, and Tetra Methyl Silane (TMS) as internal standard. 1H-NMR Spectrum shows Aromatic-Ηκ7.17-7.22(d,lH); Hj-6.37-6.38 (d,lH);Hr6.14 (s,lH); HG-4.334-4.393 (m,lH);HF-3.846- 4.007(d,2H);HE-2.273-2.349 (q,2H);HD-l.671-1.688 (s,lH); Hc-I-306-1.331 (d,3H); Hb1.055-1.105 (t,3H);HA-0.941 -2.634 (m,18H).13CMR (300MHz, CDCl3Sppm): 9.055 (CH2- CH3); 17.244; 20.002; 21.353; 23.168; 27.901; 30.622; 33.508; 34.881; 36.783; 43.642; 46.637; 47.330; 48.113; 48.417; 66.613; 70.902; 93.801; 102.732; 124.415; 129.324; 130.443; 132.472; 145.632; 153.583; 168.042; 174.853 (O-C=O); 186.208 (Cyclic C=O); 205.491 (CH-CO-CH2-OAr).
Example 3:Betamethasone 21-tosylateto Clobetasol propionate As such reaction mass used insitue for next step. Added. lithium chloride (LiCl)1.04 gm (0.0245mole). Stirred the reaction mass at 60-65°C for 5-6 hr.Reaction completion checked by TLC.After reaction completion, Added DM water (200ml). Stirred the reaction mass at 10-15°C for Ihr and Filtered washed with DM water (30mlx2).Dried in oven at 50-55°C to get white crystalline powder. Dry weight-4.42gm, (85.0%); HPLC:99.70%;mp-158-161°C. IR (KBr, cm_1):3299.62, 2976.53 (-OH); 1734.32, (C=0);1662.95 (C=C);C25H32C1F05; MSΑβ6.9Ίτη/ζ 467 [M+H];’HNMR (300MHz, CDCl35ppm): Spectrum is recorded on Varian, and Tetra Methyl Silane (TMS) as internal standard. 1H-NMR Spectrum shows AromaticHk-7.094-7. 128(d,IH); Hj-6.267-6.307 (d,lH); Hr6.066-6.076 (s,IH); H0-4.334-4.393 (m,lH); Hf-3.846-4.007 (d,2H); HE-2.273-2.349 (q,2H); Hd-I .671-1.688 (s,lH); Hc-1.306- 1.331 (d,3H); Hb-I .055-1.105 (t,3H); HA-0.941-2.634 (m,17H).13CMR (300MHz, CDCl35ppm): 8.692 (CH2-CH3); 16.693; 19.664; 21.353; 23.042; 27.568; 30.387; 36.456; 41.104; 46.547; 47.422; 71.760; 93.547; 124.307; 125.728; 127.903; 129.222; 130.443; 132.472; 145.632; 153.044; 168.312; 173.101 (O-C=O); 185.802 (Cyclic C=O); 204.602(CH-CO-CH2-C1)
SYN
Ruben Vardanyan, Victor Hruby, in Synthesis of Best-Seller Drugs, 2016
Synthesis of clobetasol propionate (27.1.13) starts from the known betamethasone 17-propionate (27.1.26), a potent glucocorticoid steroid with antiinflammatory and immunosuppressive properties, which was mesylated with methanesulfonyl chloride in pyridine to produce 9α-fluoro-11β-hydroxy-21-methylsulfonyloxy-16β-methyl 17-propionyloxypregna-1,4-diene-3,20-dione (27.1.27). The obtained product was refluxed in acetone, DMF, and dry LiCl mixture to produce the desired clobetasol propionate (27.1.13) [34] (Scheme 27.2.).


AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Medical uses
Clobetasol propionate is used for the treatment of various skin disorders including eczema, herpes labialis,[8] psoriasis, and lichen sclerosus. It is also used to treat several auto-immune diseases including alopecia areata, lichen planus (auto immune skin nodules), and mycosis fungoides (T-cell skin lymphoma). It is used as first-line treatment for both acute and chronic GVHD of the skin.[9]
Clobetasol propionate is used cosmetically by dark-skinned women for skin whitening, although this use is controversial. The U.S. Food and Drug Administration has not approved it for that purpose, and sales without a prescription are illegal in the U.S. Nonetheless, skin-whitening creams containing this ingredient can sometimes be found in ethnic beauty supply stores in New York City and on the internet. It is also sold internationally, and does not require a prescription in some countries. Whitening creams with clobetasol propionate, such as Hyprogel, can make skin thin and easily bruised, with visible capillaries, and acne. It can also lead to hypertension, elevated blood sugar, suppression of the body’s natural steroids, and stretch marks, which may be permanent.[10]
Clobetasol propionate is, along with mercury and hydroquinone, “amongst the most toxic and most used agents in lightening products.” Many products sold illegally have higher concentrations of clobetasol propionate than is permitted for prescription drugs.[11]
Contraindications
According to the California Environmental Protection Agency, clobetasol propionate should not be used by pregnant women, or women expecting to become pregnant soon, as studies with rats shows a risk of birth defects:[12]
“Studies in the rat following oral administration at dosage levels up to 50 mcg/kg per day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose. Pregnancy: Teratogenic Effects (i.e., possibility of causing abnormalities in fetuses): Pregnancy Category C: Clobetasol propionate has not been tested for teratogenicity when applied topically; however, it is absorbed percutaneously, and when administered subcutaneously it was a significant teratogen in both the rabbit and mouse. Clobetasol propionate has greater teratogenic potential than steroids that are less potent. There are no adequate and well-controlled studies of the teratogenic effects of clobetasol propionate in pregnant women. Temovate Cream and Ointment should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.”
Forms
Clobetasol propionate is marketed and sold worldwide under numerous names, including Clobex, Clob-x (Colombia), Clovate, Clobet (Biolab Thailand) Clonovate (T.O. Chemicals, Thailand), Cormax (Watson, US), Haloderm (Switzerland, by ELKO Org), Pentasol (Colombia), Cosvate, Clop (Cadila Healthcare, India), Propysalic (India), Temovate (US), Dermovate[13] (GlaxoSmithKline, Canada, Estonia, Pakistan, Switzerland, Portugal, Romania, Israel), Olux, ClobaDerm, Tenovate, Dermatovate, Butavate, Movate, Novate, Salac (Argentina), and Powercort, Lotasbat and Kloderma (Indonesia), Lemonvate (Italy), Delor (Ethiopia), Psovate (Turkey).
References
- ^ “Clobetasol Propionate Topical Ointment 0.05% Information – Drug Encyclopedia”. Kaiser Permanente.
- ^ Jump up to:a b c d e f “Clobetasol Propionate Monograph for Professionals”. Drugs.com. American Society of Health-System Pharmacists. Retrieved 13 April 2019.
- ^ Jump up to:a b c British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1210. ISBN 9780857113382.
- ^ “Clobetasol topical Use During Pregnancy”. Drugs.com. Retrieved 13 April 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 487. ISBN 9783527607495.
- ^ “The Top 300 of 2019”. ClinCalc. Retrieved 16 October 2021.
- ^ “Clobetasol – Drug Usage Statistics”. ClinCalc. Retrieved 16 October 2021.
- ^ Hull C, McKeough M, Sebastian K, Kriesel J, Spruance S (March 2009). “Valacyclovir and topical clobetasol gel for the episodic treatment of herpes labialis: a patient-initiated, double-blind, placebo-controlled pilot trial”. Journal of the European Academy of Dermatology and Venereology. 23 (3): 263–7. doi:10.1111/j.1468-3083.2008.03047.x. PMID 19143902. S2CID 205588376.
- ^ E. Fougera and Co. “CLOBETASOL PROPIONATE CREAM USP, 0.05% CLOBETASOL PROPIONATE OINTMENT USP, 0.05%<“. NIH Daily Med.
- ^ Saint Louis C (January 15, 2010). “Creams Offering Lighter Skin May Bring Risks”. New York Times.
- ^ Gbetoh MH, Amyot M (October 2016). “Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada”. Environmental Research. 150: 403–410. Bibcode:2016ER….150..403G. doi:10.1016/j.envres.2016.06.030. hdl:1866/19006. PMID 27372064.
- ^ Office of Environmental Health Hazard Assessment (August 22, 1997). “Chemicals Under Consideration For Possible Listing Via The “Formally Required To Be Labeled Or Identified” Mechanism”. California Environmental Protection Agency. Archived from the original on 2001-07-20. Retrieved 2007-05-06.
- ^ “DERMOVATE 0.05% W/V OINTMENT – Clobetasol Topical(0.05% w/v) Glaxo SmithKline Pharmaceuticals Ltd”. GNH. Retrieved 2021-07-16.
External links
- “Clobetasol propionate”. Drug Information Portal. U.S. National Library of Medicine.
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /kloʊˈbeɪtəsɒl/[1] |
| Trade names | Temovate, Clobex, Cormax, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.380 |
| Chemical and physical data | |
| Formula | C25H32ClFO5 |
| Molar mass | 466.97 g·mol−1 |
| 3D model (JSmol) | |
| (verify) | |
//////////////////Clobetasol propionate, CCI 4725, CCI-4725, GR 2/925, GR-2/925, Glucocorticoid, anti-inflammatory
[H][C@@]12C[C@H](C)[C@](OC(=O)CC)(C(=O)CCl)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C

















