Cofrogliptin
HSK 7653
- Haisco HSK 7653
- CAS 1844874-26-5
- 466.4 g/mol
- C18H19F5N4O3S
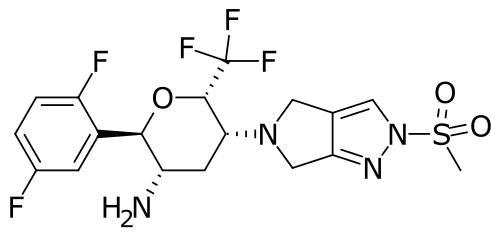
(2R,3S,5R,6S)-2-(2,5-difluorophenyl)-5-(2-methylsulfonyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-6-(trifluoromethyl)oxan-3-amine
- (2R,3S,5R,6S)-2-(2,5-Difluorophenyl)-5-(2- (methanesulfonyl)-2,6-dihydropyrrolo(3,4-C)pyrazol- 5(4H)-yl)-6-(trifluoromethyl)oxan-3-amine
- (2R,3S,5R,6S)-2-(2,5-difluorophenyl)-5-[2- (methanesulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol- 5(4H)-yl]-6-(trifluoromethyl)oxan-3-amine
- (6R)-5-Amino-2,6-anhydro-1,3,4,5-tetradeoxy-6-C-(2,5-difluorophenyl)-3-(2,6-dihydro-2-(methylsulfonyl)pyrrolo(3,4-C)pyrazol-5(4H)-yl)-1,1,1-trifluoro-D-arabino-hexitol
- (6R)-5-Amino-2,6-anhydro-1,3,4,5-tetradeoxy-6-C-(2,5-difluorophenyl)-3-[2,6-dihydro-2-(methylsulfonyl)pyrrolo[3,4-c]pyrazol-5(4H)-yl]-1,1,1-trifluoro-D-arabino-hexitol
- D-Arabino-hexitol, 5-amino-2,6-anhydro-1,3,4,5-tetradeoxy-6-C-(2,5-difluorophenyl)-3-(2,6-dihydro-2-(methylsulfonyl)pyrrolo(3,4-C)pyrazol-5(4H)-yl)-1,1,1-trifluoro-, (6R)-
- (2r,3s,5r,6s)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4h)-yl]-6-(trifluoromethyl)-tetrahydro-2h-pyran-3-amine
APPROVALS 2024, CHINA 2024, Haisco Pharmaceutical Group Co, Beichangping, DIABETES
Cofrogliptin (developmental name HSK7653) is a long-acting DPP4 inhibitor dosed once every two weeks.[1][2][3][4]
Cofrogliptin (HSK7653) (compound 2), a tetrahydropyran derivative, is a potent oral dipeptidyl aminopeptidase 4 (DPP-4) inhibitor with Long-acting antidiabetic efficacy. Cofrogliptin (compound 2) has a great potential for type 2 diabetes mellitus (T2DM) .
SYN
J Med Chem. 2020 Jul 9;63(13):7108-7126
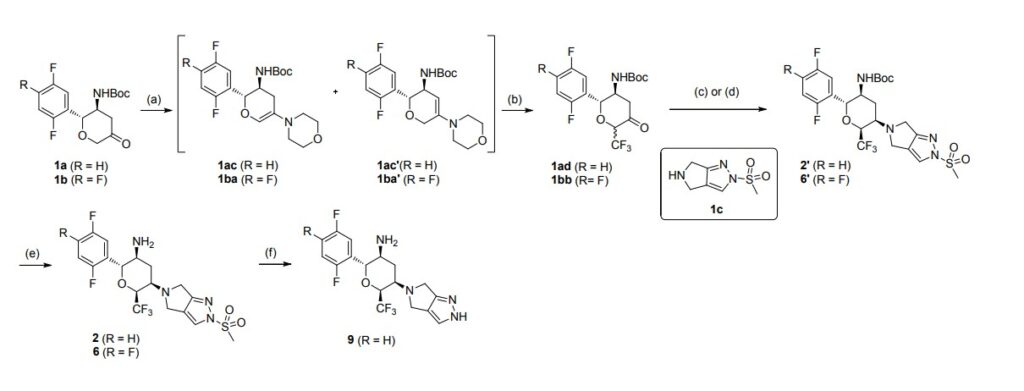
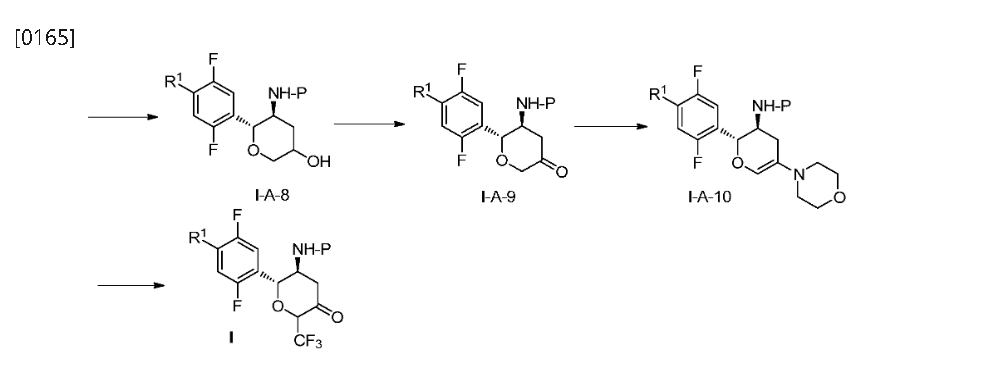
aReagents and conditions: (a) morpholine, toluene, reflux in Dean-Stark appartus; (b)
Umemoto’s reagent, DMAP, DMAc; (c) step 1: 1c, toluene, reflux; step 2: NaBH(OAc)3, CH3COOH, 1,2-DCE; (d) step 1: 1c, CHCl3, reflux in Dean-Stark apparatus; step 2:
NaBH(OAc)3, CH3COOH, 1,2-DCE; (e) TFA, DCM; (f) t-BuOK, THF
Step 2: To a stirred solution of tert-butyl N-[(2R,3S,5R,6S)-2-(2,5-difluorophenyl)-5-
(2-methylsulfonyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)-6-
(trifluoromethyl)tetrahydropyran-3-yl]carbamate (2′) (407.5 mg, 0.72 mmol) in DCM (6
mL) was added CF3COOH (2 mL) under nitrogen at 0 ℃. After the addition, the reaction
mixture was allowed to warm to room temperature and stirred for 2 h. The reaction mixture
was quenched with a saturated solution of Na2CO3 (15 mL), and extracted with DCM (15
mL × 2). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo.
The residue was purified by flash column chromatography (Eluent: DCM/MeOH = 80:1–
30:1) to afford the desired product 2 (301.9 mg, yield: 90%). White solid. Mp: 150.1–152.0
℃. [α]D20 = +17.6 (c = 2.000 in MeOH). Rf= 0.40 (1:15 MeOH/CH2Cl2, TLC).
1H NMR
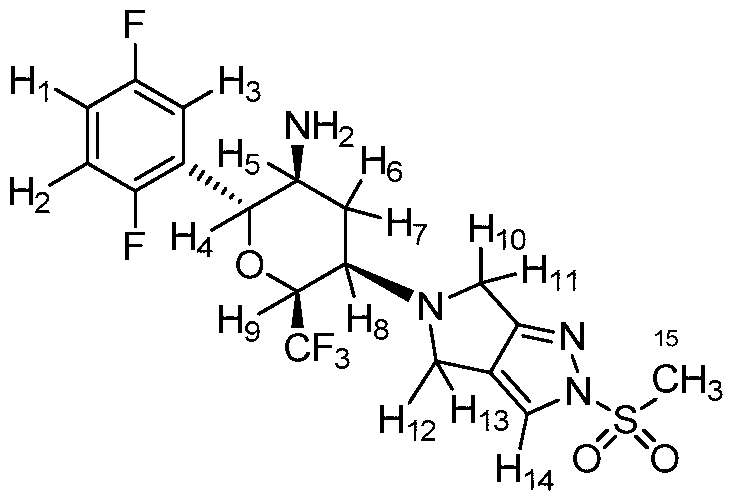
(400 MHz, CDCl3) δ = 7.71 (s, 1H), 7.20 – 7.12 (m, 1H), 7.10 – 6.97 (m, 2H), 4.63 (d, J =
10.0 Hz, 1H), 4.49 – 4.38 (m, 1H), 4.07 – 3.97 (m, 2H), 3.93 – 3.81 (m, 2H), 3.53 – 3.42
(m, 1H), 3.29 (s, 3H), 3.01 – 2.91 (m, 1H), 2.45 – 2.35 (m, 1H), 2.07 – 1.93 (m, 1H), 1.19
(br. s, 2H). 13C NMR (100 MHz, CDCl3) δ = 163.6, 159.1 (dd, J = 2.3 Hz, 235.8 Hz), 156.6
SYN
https://www.sciencedirect.com/science/article/abs/pii/S0223523424003441
SYN
WO2015192701
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015192701&_cid=P20-MEQV3M-18104-1
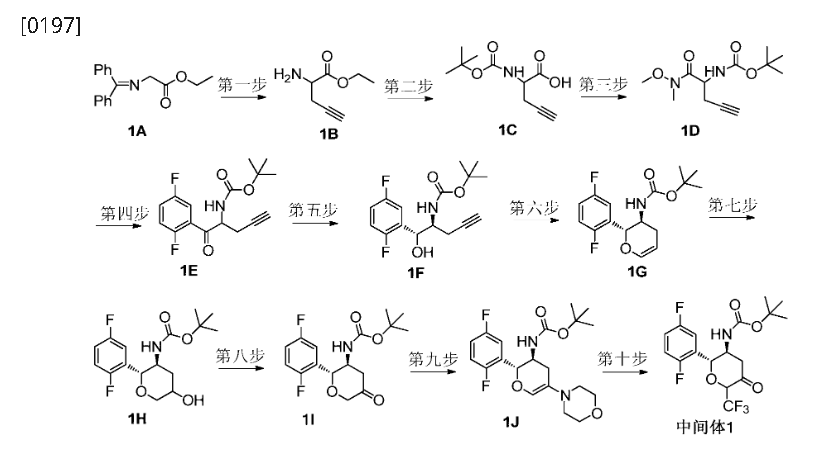
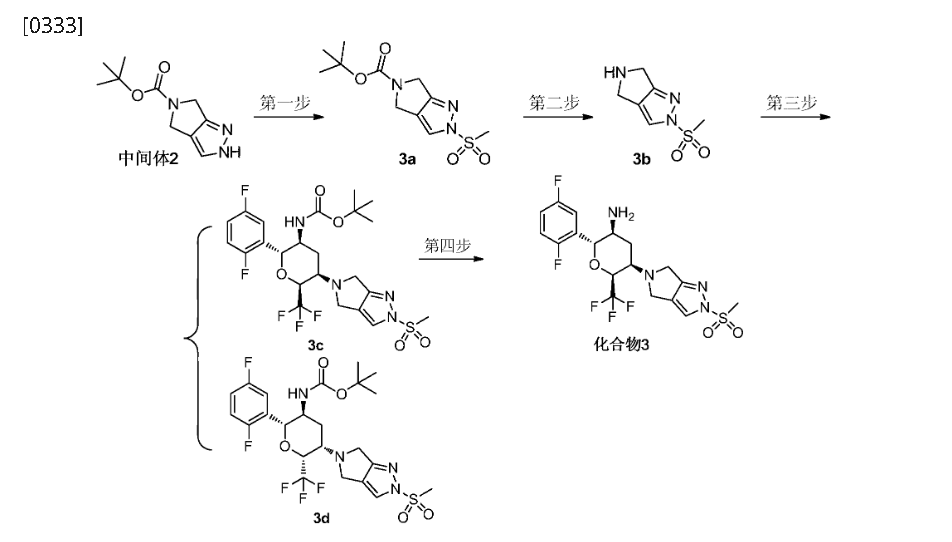
Step 4: (2R,3S,5R,6S)-2-(2,5-difluorophenyl)-5-(2-(methylsulfonyl)-pyrrolo[3,4]pyrazol-5(2H,4H,6H)-yl)-6-(trifluoromethyl)tetrahydro-2H-pyran-3-amine (Compound 3)
[0345]
(2R,3S,5R,6S)-2-(2,5-difluorophenyl)-5-(2-(methylsulfonyl)pyrrolo[3,4-c]pyrazol-5(2H,4H,6H)-yl)-6-(trifluoromethyl)tetrahydro-2H-pyran-3-amine
[0346]3c (410 mg, 0.72 mmol) was dissolved in 6 mL of dichloromethane and 2 mL of trifluoroacetic acid and stirred at room temperature for 1 hour. After completion, saturated aqueous sodium bicarbonate (30 mL) was added to quench the reaction. After separation, the aqueous phase was extracted with ethyl acetate (30 mL x 2). The combined organic phases were dried over anhydrous sodium sulfate, and concentrated. Purification by silica gel column chromatography (dichloromethane/methanol (v/v) = 30:1) afforded compound 3 (250 mg, 75% yield) as a white powder.
[0347]MS m/z(ESI): 467.1[M+1];
[0348]
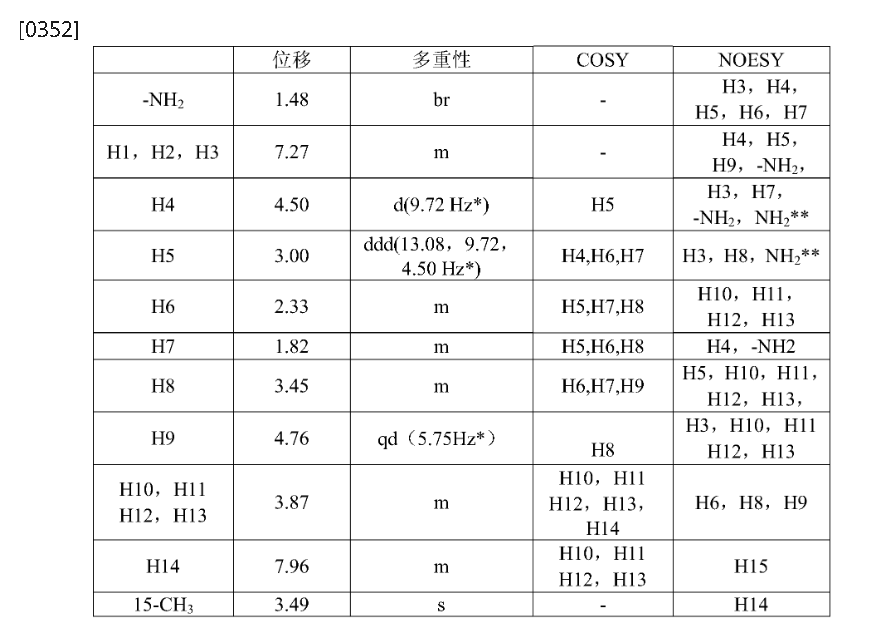
1H NMR(400MHz,DMSO-d 6):δ7.96(m,1H),7.35–7.04(m,3H),4.86–4.63(qd,1H),4.50(d,1H),3.95(dd,2H),3.78(dd,2H),3.49(s,3H),3.45(m,1H),3.00(ddd,1H),2.33(m,1H),1.82(m,1H),1.48(br,2H)。
SYN
Cofrogliptin, developed by Haisco Pharmaceutical Group Co., Ltd., is a novel, ultra-long-acting dipeptidyl peptidase-4 (DPP-4) inhibitor designed for the treatment of T2DM. It is marketed under the brand name (Beichangping). In 2024, the NMPA approved Cofrogliptin for improving blood glucose control in adult patients with T2DM [59].Cofrogliptin acts pharmacologically by inhibiting DPP-4, an enzyme tasked with degrading incretin hormones like glucagon-like peptide-1(GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By obstructing the degradation of these hormones, it amplifies their activity. This leads to a glucose-dependent rise in insulin secretion and a
corresponding decrease in glucagon release, which in turn improves glycemic control. The clinical efficacy of Cofrogliptin was demonstrated in Phase III, randomized, double-blind, non-inferiority trial
(NCT04556851), where its efficacy and safety were compared to those of daily linagliptin in patients with T2DM whose blood sugar was not well-controlled by metformin. The study reported that Cofrogliptin
administered once every two weeks achieved a reduction in HbA1c comparable to that of daily linagliptin, with a mean decrease of approximately 0.96 % over 24 weeks. Regarding toxicity, Cofrogliptin
was generally well-tolerated [60,61]. The incidence of hypoglycemia was low, and no severe hypoglycemic events directly attributed to the drug were reported.
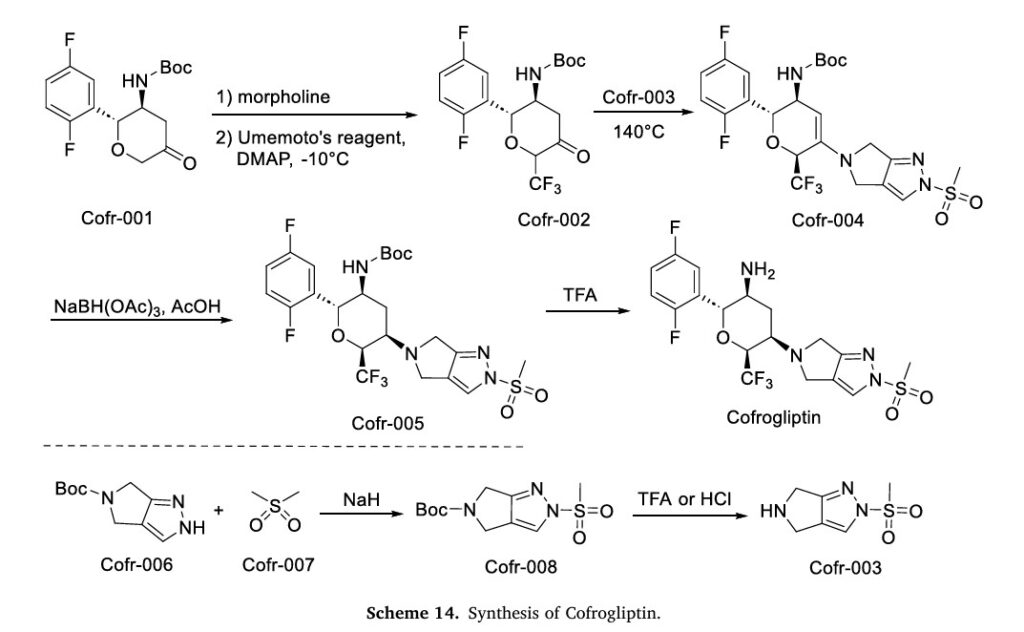
The synthesis of Cofrogliptin, illustrated in Scheme 14, initiates with trifluoromethylation of Cofr-001 via oxidation, affording Cofr-002 [62]. Nucleophilic addition of Cofr-003 to Cofr-002 yields Cofr-004, followed by NaBH(OAc)3 reduction to Cofr-005. TFA-mediated deprotection of Cofr-005 ultimately delivers Cofrogliptin. Concurrently, Cofr-006 undergoes nucleophilic substitution with Cofr-007 to form Cofr-008, whose deprotection regenerates Cofr-003
[59] L. Gao, F. Bian, T. Pan, H. Jiang, B. Feng, C. Jiang, J. Sun, J. Xiao, P. Yan, L. Ji,
Efficacy and safety of cofrogliptin once every 2 weeks in Chinese patients with type
2 diabetes: a randomized, double-blind, placebo-controlled, phase 3 trial, Diabetes
Obes Metab 27 (2025) 280–290.
[60] C. Cui, F. Cao, I.I. Kong, Q. Wu, F. Li, H. Li, D. Liu, A model-informed approach to
accelerate the clinical development of cofrogliptin (HSK7653), a novel ultralong-
acting dipeptidyl peptidase-4 inhibitor, Diabetes Obes Metab 26 (2024) 592–601.
[61] Q. Ren, L. Li, X. Su, X. Hu, G. Qin, J. Han, Y. Liu, J. Wang, L. Ji, Cofrogliptin once
every 2 weeks as add-on therapy to metformin versus daily linagliptin in patients
with type 2 diabetes in China: a randomized, double-blind, non-inferiority trial,
Diabetes Obes Metab 26 (2024) 5013–5024.
[62] C. Zhang, J. Wang, C. Li, Y. Wei, Amino Pyranoid Ring Derivative as DPP-IV
Inhibitor and Its Preparation, 2015. WO2015192701A1.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Ji, Linong; Bian, Fang; Pan, Tianrong; Jiang, Hongwei; Jiang, Chengxia; Ren, Qian (20 June 2023). “55-OR: HSK7653, a Novel Ultralong-Acting DPP-4 Inhibitor, as Monotherapy in Patients With Type 2 Diabetes—A Randomized, Double-Blind, Placebo-Controlled Phase III Trial”. Diabetes. 72 (Supplement_1). doi:10.2337/db23-55-OR. S2CID 259433641.
- Zhang, Miao; Zhang, Shudong; Yu, Zhiheng; Yao, Xueting; Lei, Zihan; Yan, Pangke; Wu, Nan; Wang, Xu; Hu, Qin; Liu, Dongyang (October 2023). “Dose decision of HSK7653 oral immediate release tablets in specific populations clinical trials based on mechanistic physiologically-based pharmacokinetic model”. European Journal of Pharmaceutical Sciences. 189 106553. doi:10.1016/j.ejps.2023.106553. PMC 10485820. PMID 37532063.
- Liu, Yang; Yan, Shuai; Liu, Jie; Liu, Hongzhong; Song, Ling; Yao, Xueting; Jiang, Ji; Li, Fangqiong; Du, Ke; Liu, Dongyang; Hu, Pei (May 2023). “Development and validation of an HPLC coupled with tandem mass spectrometry method for the determination of HSK7653, a novel super long-acting dipeptidyl peptidase-4 inhibitor, in human plasma and urine and its application to a pharmacokinetic study”. Biomedical Chromatography. 37 (5): e5607. doi:10.1002/bmc.5607. PMID 36802077. S2CID 257048524.
- Bai, Nan; Wang, Jin; Liang, Wenxin; Gao, Leili; Cui, Wei; Wu, Qinghe; Li, Fangqiong; Ji, Linong; Cai, Yun (6 November 2023). “A Multicenter, Randomized, Double-Blind, Placebo-Controlled, and Dose-Increasing Study on the Safety, Tolerability and PK/PD of Multiple Doses of HSK7653 by Oral Administration in Patients with Type 2 Diabetes Mellitus in China”. Diabetes Therapy. 15 (1): 183–199. doi:10.1007/s13300-023-01496-0. PMC 10786778. PMID 37930584.
| Clinical data | |
|---|---|
| Other names | HSK7653 |
| Legal status | |
| Legal status | Investigational |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1844874-26-5 |
| PubChem CID | 118613788 |
| ChemSpider | 115037226 |
| UNII | LH4G6K6NKP |
| ChEMBL | ChEMBL4646510 |
| Chemical and physical data | |
| Formula | C18H19F5N4O3S |
| Molar mass | 466.43 g·mol−1 |
- [1]. International Nonproprietary Names for Pharmaceutical Substances (INN)[2]. Chen Zhang, et al. Design, Synthesis, and Evaluation of a Series of Novel Super Long-Acting DPP-4 Inhibitors for the Treatment of Type 2 Diabetes. J Med Chem. 2020 Jul 9;63(13):7108-7126. [Content Brief]
///////Cofrogliptin, APPROVALS 2024, CHINA 2024, Haisco Pharmaceutical Group Co, Beichangping, DIABETES, HSK 7653, Haisco HSK 7653, 1844874-26-5














