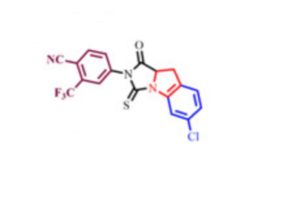
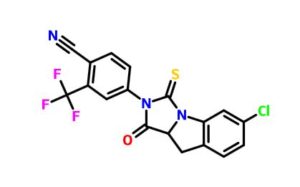
4-(6-chloro-1-oxo-3-thioxo-9,9a-dihydro-1H-imidazo[1,5-a]indol-2(3H)-yl)-2-(trifluoromethyl)-benzonitrile
WILL BE UPDATED………
Prostate cancer, one of the most malignant tumors worldwide, is the second leading cause of cancer deaths among men in America . Although androgen deprivation therapy (ADT) has been proved to be effective initially, the tumor will eventually progress and develop into the lethal castration resistant prostate cancer (CRPC) . The androgen receptor (AR) is a ligand-dependent transcription factor belonging to the nuclear receptor superfamily and plays a critical role in the progression of normal prostate cells. However, overexpression of AR was found in most CRPC, which is essential for CRPC to adapt to the low levels of androgens. As AR contributes significantly to the resistance to castration, it has been recognized as an attractive target for the treatment of CRPC
Reagents and conditions: (i) HNO3 , H2SO4 , -5 oC, 3 h; (ii) SOCl2 , MeOH, reflux, 12 h; (iii) H2 , Pd/C, MeOH, rt, 12 h; (iv) (CH3CO)2O, TEA, 50 oC, 6 h; (v) H2 , Pd/C, MeOH, rt, 12 h; (vi) acetone, HCl (6 mol/L), -10 oC, 0.5 h, NaNO2 , H2O, -10 oC, 1 h, CuCl/CuBr/KI, 0 oC, 3 h; (vii) HCl, 50 oC, 3 h; (viii) SOCl2 , MeOH, reflux, 12 h; (ix) 2, DMF, TEA, 60 oC, 1 h.
4-(6-chloro-1-oxo-3-thioxo-9,9a-dihydro-1H-imidazo[1,5-a]indol-2(3H)-yl)-2-(trifluoromethyl)-benzonitrile (48c). It was obtained as a yellow solid
m.p. 220-222 oC;
1H-NMR (300 MHz,DMSO-d6): δ 8.40 (d, J = 8.1 Hz, 1H, Ar-H), 8.19 (s, 1H, Ar-H), 8.02-7.92 (m, 2H, Ar-H), 7.49-7.46 (m, 1H, Ar-H), 7.34-7.32 (m, 1H, Ar-H), 5.56 (t, J = 9.6 Hz, 1H, -CH-), 3.58 (d, J = 9.6 Hz, 2H, -CH2-) ppm;
13C-NMR (75 MHz, DMSO-d6): δ 184.1, 172.1, 142.3, 138.5, 136.8, 134.7, 131.9, 131.5, 128.0, 126.1 (q, J = 267.9 Hz, CF3), 117.2, 115.4, 66.9, 39.9 ppm;
IR (KBr): 3094, 2232, 1763, 1607, 1499, 1270, 1136, 1052, 998, 786 cm-1;
HRMS (ESI): m/z, calculated for C18H9ClF3N3OS 408.0180 (M + H)+ , found 408.0173.
Paper
A series of indoline thiohydantoin derivatives were synthesized and evaluated in vitro.The most potent compound 48c shows comparable ability with enzalutamide in proliferation inhibition of LNCaP cells.Compound 48c has less cytotoxic to AR-negative cells compared with Enzalutamide.
The bicalutamide-resistant mechanism was clarified and overcome by compound 48c.
Abstract
A novel scaffold of indoline thiohydantoin was discovered as potent androgen receptor (AR) antagonist through rational drug designation. Several compounds showed good biological profiles in AR binding and higher selective toxicity than enzalutamide toward LNCaP cells (AR-rich) versus DU145 cells (AR-deficient). In addition, the docking studies supported the rationalization of the biological evaluation. Among these compounds, the representative compound 48c exhibited the strongest inhibitory effect on LNCaP growth and also acted as a competitive AR antagonist. Further preliminary mechanism study confirmed that 48c exerted its AR antagonistic activity through impairing AR nuclear translocation. All these results indicated that the novel scaffold compounds demonstrated AR antagonistic behaviour and promising candidates for future development were identified.
Available online 22 October 2016
Design and synthesis of indoline thiohydantoin derivatives based on enzalutamide as antiproliferative agents against prostate cancer
- Department of Medicinal Chemistry, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009, PR China
- zhiyuli@cpu.edu.cn
http://www.sciencedirect.com/science/article/pii/S0223523416309114
http://dx.doi.org/10.1016/j.ejmech.2016.10.049
////////Prostate cancer, Androgen receptor, Antagonist, Indoline thiohydantoin derivatives, indoline thiohydantoin derivatives, enzalutamide, antiproliferative agents, prostate cancer
c1c(cc(c(c1)C#N)C(F)(F)F)N2C(C3Cc4ccc(cc4N3C2=S)Cl)=O