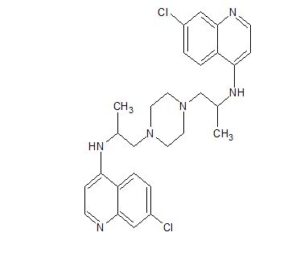
CORRECT STR OF Dichlorquinazine
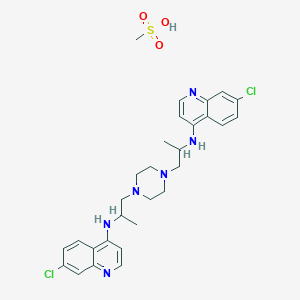
7-chloro-N-[1-[4-[2-[(7-chloroquinolin-4-yl)amino]propyl]piperazin-1-yl]propan-2-yl]quinolin-4-amine;methanesulfonic acid
- 1,4-Piperazinediethanamine, N,N’-bis(7-chloro-4-quinolinyl)-α,α’-dimethyl- (9CI)
- Quinoline, 4,4-[1,4-piperazinediylbis[(1-methylethylene)imino]]bis[7-chloro- (7CI)
- Quinoline, 4,4′-[1,4-piperazinediylbis[(1-methylethylene)imino]]bis[7-chloro- (8CI)
- N1,N4-Bis(7-chloro-4-quinolinyl)-α1,α4-dimethyl-1,4-piperazinediethanamine
- 1,4-Bis[2-(7-chloro-4-quinolylamino)propyl]piperazine
- Bis[(chloro-7”-quinolyl-4”)amino-2′-propyl]-1,4-piperazine
- Dichlorquinazine
- N,N’-Bis(7-chloro-4-quinolyl)-α,α’-dimethylpiperazine-1,4-diethylamine
- NSC 129790
- RP 12278
- WR 3863
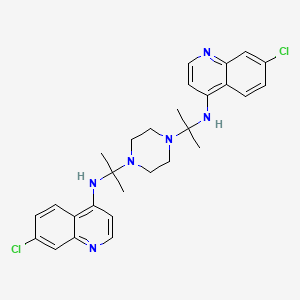
WRONG STRUCTURE
WRONG STRUCTURE
Dichlorquinazine
- BRN 0867697
- Dichlorquinazine
- EINECS 234-130-6
- NSC 129790
- RP 12278
- UNII-HT3GAD2SCM
- WR 3863
cas 10547-40-7
C28H32Cl2N6, mw
| 523.5 |
7-chloro-N-[2-[4-[2-[(7-chloroquinolin-4-yl)amino]propan-2-yl]piperazin-1-yl]propan-2-yl]quinolin-4-amine
VARIANT
RN: 23256-65-7
Molecular Formula, C28-H32-Cl2-N6.C-H4-O3-S, Molecular Weight, 619.6144
-
RP-12278 mesylate
-
WR-3863 mesylate
-
Quinoline, 4,4′-(1,4-piperazinediylbis((1-methylethylene)imino))bis(7-chloro-, tetramethanesulfonate bis((7-chloro-4”-quinolyl)-2′-aminopropyl)-1,4-piperazine methanesulfonate

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
PATENTS
BE 626239
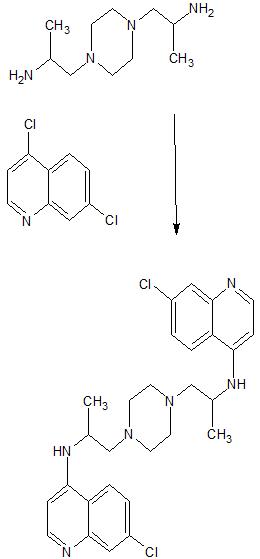
4-(Chloro or alkoxy)quinolines are treated with a 1,4-bis(aminoalkyl)piperazine to give the title compds. which can be used as antiinflammatory agents and as amebicides. Thus, a mixt. of 16.3 g. 4-chloroquinoline, 10 g. 1,4-bis(3-aminopropyl)piperazine, 55 g. PhOH, and 0.2 g. NH4Cl is heated 5 hrs. at 175°, poured into a mixt. of 500 ml. H2O and 100 ml. NaOH (d. 1.33), filtered, the ppt. is treated with a mixt. of 80 ml. H2O and 20 ml. NaOH, the mixt. filtered, and the ppt. washed with 500 ml. H2O and dried to give 15.9 g. 1,4-bis[3-(4-quinolyl)aminopropyl]piperazine, m. 210°(MeOH-H2O). Similarly prepd. are the following I: n, R, R1, R2, X, Y, m.p.; 2, H, H, H, MeO, H, 245° (HCONMe2); 2, H, H, H, H, SO2NMe2, 271° (HCONMe2); 2, H, H, H, H, CF3, 293° (HCONMe2); 3, Me, H, H, H, H, ∼100°; 3, Me, Ac, H, H, H, -(1); 3, Me, H, H, MeOH, 180° and 190°; 3, Me, Ac, H, MeO, H, -(2); 1, Me, H, Me, H, Cl, 264°; 2, H, H, H, Cl, H, 264° (BuOH); 1, Me, H, H, H, CF3, 240° (MeCOEt); 2, H, H, H, H, MeO, 200° (EtOH); 2, H, H, Me, H, MeO, 216° (EtOH); 3, Me, H, H, H, MeO, 218° (CH2Cl2); (1) bis(acid maleate) m. 155° (iso-PrOH), (2) bis(acid maleate) m. 155° The following II were also prepd.: n, R, R1, R2, m.p.; 1, Me, A(R = R1 = X = Y = H,Z =Cl), A(R = R1 = X = Z = H,Y = Cl), 208-10° (HCONMe2); 1, Me, A(R = R1 = X = Y= H, Z = Cl), A(R = R1 = X = Y = H,Z = MeO), 206-8° (HCONMe2); 1, Me, A(R1 = X = Y = H, R = 4-ClC6H4, Z = Cl), A(R = R1 = X = Y = H,Z = Cl, 230-2° (HCONMe2) The following III were prepd.: n, R, m, R1, R2, m.p.; 3, Me, 1, H, A(R = R1 = X = Y = H, Z= Cl), 190-1° and 213-15°; 2, H, 2, H, A(R = X = Y = H, R1 = Me, Z =Cl), 198° (PrOH); 3, Me, 2, H, A(R = R1 = X = Y = H,Z = Cl), 160-2°; 1, Me, 1, H, A(R = R1 = X = Y = H,Z = Cl), 178°; 1, Me, 1, Me, A(R1 = X = Z = H,R = Me, Y =AcNH), 330° (decompn.) (EtOH); 2, H, 2, H, A(R1 = X = Y = H,R = 4-ClC6H4,Z = Cl), 320-1° (HCONMe2); 2, H, 2, H, A(R = Y = Z = H, R1 = Me, X = Cl) 96° (iso-PrOH); 1, Me, 1, Me, A(R = R1 = X = Z = H, Y = Cl), 220° and 246-8°; 1, Me, 1, Me, A(R1 = X = Z = H, R = Me, Y = NH2), 305° (EtOH-H2O); 1, Me, 1, Me, A(R1 = X = Z = H, R = Me, Y = MeO, 244° (EtOH) Also prepd. were (m.p. given): 1,4-bis[2-(7-chloro-4-quinolylamino)propyl]hexahydro-1,4-diazepine, 169°; 1-[5-(7-chloro-4-quinolylamino)-2-pentyl]-4-[2-(7-chloro-4-quinolylamino)propyl] piperazine, 210-12°(HCONMe2); 1,4-bis[3-(7-chloro- 4-quinolylamino)propyl] hexahydro-1,4-diazepine, 186° (HCONMe2). The following were prepd. (m.p. and optical rotation given):L(+)-1,4-bis[2-(7-chloro-4-quinolylamino)propyl]piperazine, 250-1°, [α]23.5D 382° ± 1° (c 4, 50:50 MeOH-H2O); D(-)-1,4- bis[2-(7-chloro-4-quinolylamino)propyl] piperazine, 250-1°, [α]25D -382.5° ± 1° (c 4, 50:50 MeOH-H2O); DL-1,4-bis[2-(7-chloro-4-quinolylamino)propyl]piperazine (IV), 266-8°, -; meso-1,4-bis [2-(7-chloro-4-quinolylamino)propyl] piperazine (V), 270-1° (HCONMe2), -; equimol. mixt. of IV and V, 250-2°, -; 1,4-bis[2-(6-chloro-4-quinolylamino)propyl]piperazine-form A (VI-form A), 227° -; VI-form B, 110° and 245°, -. Also prepd. are the following intermediates of the general formula VII (R = H) (X, Y, Z, and m.p. given): OH, H, SO2NMe2, ∼288°; Cl, H,SO2NMe2, 170°; HO(CH2)3CHMeNH, H, H, 158° (EtOH); AcO(CH2)3CHMeNAc, H, H, -; HO(CH2)3CHMeNAc, H, H, -; MeSO3(CH2)3CHMeNAc, H, H, -; N-(5-piperazino-2-pentyl)acetamido, H, H, -; HO(CH2)3CHMeNH, MeO, H, -; AcO(CH2)3CHMeNAc, MeO, H, -; HO(CH2)3CHMeNAc, MeO, H, -; MeSO3(CH2)3CHMeNAc, MeO, H, -; N-(5-piperazino-2-pentyl)acetamido, MeO, H, -; Me(HOCH2)CH, H, Cl, 210°; Me(ClCH2)CH, H, Cl, 148-50°; Me(HOCH2)CH, Cl, H, 192°; Me(ClCH2)CH, Cl, H, 142°; Me(HOCH2)CH, H, MeO, 170°; Me(ClCH2)CH, H, MeO, 160°. Also prepd. were (m.p. given): VII (R = CO2Et, X = OH, Y = H, Z = SO2NMe2), ∼335°; VII (R = CO2H, X = OH, Y = H, Z = SO2HMe2), 310° (decompn.); 1,4-bis(2-oxopropyl)hexahydro-1,4-diazepine, -; 1,4-bis(2-oximinopropyl)hexahydro-1,4-diazepine, 180-1°; 1,4-bis(2-aminopropyl)hexahydro-1,4-diazepine, -; 1,4-bis(2-cyanoethyl)-hexahydro-1,4-diazepine, -. The following were prepd. (m.p. and optical rotation given): L(+)-4-(3-hydroxy-2-propylamino)-7-chloroquinoline, 223-4°, [α]24D 28.5° ± 2° (c 1, EtOH); L(+)-4-(3-chloro-2-propylamino)-7-chloroquinoline, 146-7°, [α]24D 103 ± 1° (c 2, EtOH); L(+)-4-(3-piperazino-2-propylamino)-7-chloroquinoline, 128-30°, [α]23D 139 ± 1° (c 2, EtOH); D(-)-4-(3-hydroxy-2-propylamino)-7-chloroquinoline, 223-4°, [α]25D – 31 ± 2° (c 1, EtOH); D(-)-4-(3-chloro-2-propylamino)-7-chloroquinoline, 147-8°, [α]24D -101 ± 1° (c 2, EtOH); D(-)-4-(3-piperazino-2-propylamino)-7-chloroquinoline, 131-2°, [α]23D -137 ± 1° (c 2, EtOH)
PATENT
FR CAM42 19631007.
Piperazines (I) are antiinflammatory and anthelmintic agents. A mixt. of 8.25 g. MeCH(NH2)CH2OH, 19.8 g. 4,6-dichloroquinoline, and 55 g. PhOH is heated to give 16.0 g. 6-chloro-4-[(3-hydroxy-2-propyl)-amino]quinoline (II), m. 192°. II (14.0 g.) is treated with a soln. of 10.6 g. SOCl2 in 40 ml. CHCl3 to give 12.5 g. 6-chloro-4-[(3-chloro-2-propyl)amino]quinoline (III), m. 142°. A mixt. of 13.2 g. 1-[2-(7-chloro-4-quinolylamino)propyl]piperazine, 11.0 g. III, 6.4 g. NaI, 2.3 g. anhyd. Et3N, and 200 ml. AcEt is refluxed 18 hrs., the solvent is distd. in vacuo, and the residue is taken up in 100 ml. MeOH. The mixt. is made alk. with 110 ml. NaOH (d. 1.33), poured into 1000 ml. H2O, and the ppt. that forms is filtered off, washed with H2O, and recrystd. in HCONMe2 to give 11.0 g. 1-[2-(7-chloro-4-quinolylamino)propyl]-4-[2-(6-chloro-4-quinolylamino)propyl]piperazine, m. 208-10°. Similarly prepd. are the following I (R, m, R1, n, R2, R3, R4, and m.p. given): H, 2, H, 2, H, MeO, H, 245°; H, 2, H, 2, H, H, SO2NMe2, 271°; H, 2, H, 2, H, H, CF3, 293°; Me, 3, Me, 3, H, MeO, H, 180° and 190°; Me, 3, H, 1, H, H, Cl, 190-1° and 213-15°; H, 2, H, 2, H, Cl, H, 264°; Me, 1, Me, 1, H, H, CF3, 240°; H, 2, H, 2, H, H, MeO, 200°; Me, 3, H, 2, H, H, Cl, 160-2°; Me, 1, H, 1, H, H, Cl, 178°; Me, 1, Me, 1, Me, AcNH, H, 330°; H, 2, H, 2, p-ClC6H4, H, Cl, 320-1°; Me, 1, Me, 1, H, Cl, H, 227° (form A); Me, 1, Me, 1, H, Cl, H, 110° and 245° (form B); H, 3, H, 3, H, H, Cl, 239-41°; Me, 1, Me, 1, Me, NH2, H, 305°; Me, 1, Me, 1, Me, MeO, H, 244°; Me, 3, Me, 3, Me, 3, H, H, MeO, 218°; H, 3, H, 3, H, H, Cl, 240-2°. Also prepd. are (m.p. given): 1,4-bis[2-(7-chloro-4-quinolylamino)propyl]hexahydrodiazepine, 169°; 2,5-dimethyl-1,4-bis[2-(7-chloro-4-quinolylamino)propyl)piperazine, 264°; 1-[5-(7-chloro-4-quinolylamino [-2-pentyl]-4-[2-(7-chloro -4-quinolylamino)propyl]piperazine, 210-12°; 2,5-dimethyl-1,4-bis[3-(7-methoxy-4-quinolylamino)propyl]piperazine, 216°; 1,4-bis[3-(3-methyl-7-chloro-4-quinolylamino)propyl] piperazine, 198°; 1,4-bis[3-(7-chloro-4-quinolylamino)propyl]hexahydrodiazepine, 186°; 1-[2(7-chloro-4-quinolylamino)propyl]-4-[2-(7-methoxy-4-quinolylamino)propyl]piperazine, 206-8°; 1,4-bis[3-(3-methyl-5-chloro-4- quinolylamino)propyl]piperazine, 96°; 1 – [2 -[2 -(p – chlorophenyl)- 7- chloro- 4- quinolylamino]propyl] -4 – [2 – (7 – chloro – 4-quinolylamino)propyl]piperazine, 230-2°; L(+) 1,4-bis[2-(7-chloro-4-quinolylamino)propyl]piperazine, 250-1°, [α]23.5D + 382° ± 1° (c 4, 50/50 MeOH-H2O); L(+)-7-chloro-4-(3-hydroxy-2-propylamino)quinoline, 223-4°, [α]24D 28.5° ± 2° (c 1, EtOH); L(+)-7-chloro-4-(3-chloro-2-propylamino)quinoline, 146-7°, [α]24D 103° + 1° (c 2, EtOH); L(+)-7-chloro-4-(3-piperazino-2-propylamino)quinoline 128-30°, [α]23D 139° ± 1° (c 2, EtOH); D(–)-1,4-bis[2-(7-chloro-4-quinolylamino)propyl]piperazine, 250-1°, [α]25D -382° ± 1° (c 4, 50:50 MeOH-H2O); meso- 1,4 – bis [2 – (7 – chloro – 4 – quinolylamino)propyl] piperazine, 270-1°.
Patent Information
BE 612207
|
Publication Number
|
Title
|
Priority Date
|
Grant Date
|
|---|---|---|---|
| US-2016045487-A1 | Compositions and methods for treating neuropathy | 2013-03-27 | |
| WO-2014160811-A1 | Compositions and methods for treating neuropathy | 2013-03-27 | |
| AU-2014234258-A1 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | |
| AU-2014234258-B2 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | 2019-02-14 |
| CA-2907628-A1 | Piperaquine microcapsules and compositions containing them | 2013-03-22 |
|
Publication Number
|
Title
|
Priority Date
|
Grant Date
|
|---|---|---|---|
| EP-2976069-A1 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | |
| EP-2976069-B1 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | 2020-05-06 |
| US-2014322296-A1 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | |
| US-2016045447-A1 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | |
| US-9668979-B2 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | 2017-06-06 |
|
Publication Number
|
Title
|
Priority Date
|
Grant Date
|
|---|---|---|---|
| WO-2014147242-A1 | Piperaquine microcapsules and compositions containing them | 2013-03-22 | |
| AU-2009215107-A1 | Treatments for neuropathy | 2008-02-12 | |
| AU-2009215107-B2 | Treatments for neuropathy | 2008-02-12 | 2013-05-09 |
| AU-2013203934-A1 | Treatments for neuropathy | 2008-02-12 | |
| CA-2714676-A1 | Treatments for neuropathy | 2008-02-12 |
|
Publication Number
|
Title
|
Priority Date
|
Grant Date
|
|---|---|---|---|
| CA-2714676-C | Treatments for neuropathy | 2008-02-12 | 2015-04-14 |
| EP-2240177-A2 | Treatments for neuropathy | 2008-02-12 | |
| US-2009203735-A1 | Treatments for neuropathy | 2008-02-12 | |
| US-2011086878-A1 | Treatments for Neuropathy | 2008-02-12 | |
| US-2016058749-A1 | Treatments for neuropathy | 2008-02-12 |
////////////////Dichlorquinazine, BRN 0867697, Dichlorquinazine, EINECS 234-130-6, NSC 129790, RP 12278, UNII-HT3GAD2SCM, WR 3863
CC(C)(NC1=C2C=CC(=CC2=NC=C1)Cl)N3CCN(CC3)C(C)(C)NC4=C5C=CC(=CC5=NC=C4)Cl
WRONG
CC(CN1CCN(CC(C)
AND
Clc1ccc2c(c1)nccc2NC(C)CN1CCN(CC(C)Nc2ccnc3cc(Cl)ccc32)CC1
CORRECT