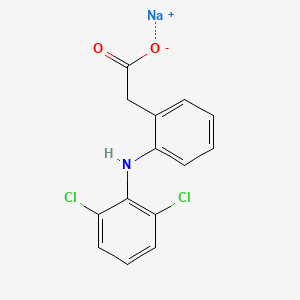
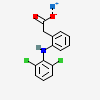
Diclofenac Sodium
15307-79-6; Sodium diclofenac; Diclofenac sodium salt; Voltaren; Solaraze
| Molecular Formula: | C14H10Cl2NNaO2 |
|---|---|
| Molecular Weight: | 318.129 g/mol |
Diclofenac, sold under the trade names Voltaren among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammatory diseases such as gout.[3] It is taken by mouth or applied to the skin.[3] Improvements in pain typically occur within half an hour and last for as much as eight hours.[3] It is also available in combination with misoprostol in an effort to decrease stomach problems.[4]
Common side effects include abdominal pain, gastrointestinal bleeding, nausea, dizziness, headache, and swelling.[3] Serious side effects may include heart disease, stroke, kidney problems, and stomach ulceration.[4][3] Use is not recommended in the third trimester of pregnancy.[3] It is likely safe during breastfeeding.[4] It is believed to work by decreasing the production of prostaglandin.[5] It blocks both cycloxygenase-1 (COX-1) and cycloxygenase-2 (COX-2).[3]
Diclofenac was patented in 1965 by Ciba-Geigy and came into medical use in the United States in 1988.[3][6] It is available as a generic medication.[3] In the United States the wholesale cost per dose is less than US$0.15 as of 2018.[7] In 2016 it was the 78th most prescribed medication in the United States with more than 9 million prescriptions.[8] It is available as both a sodium and a potassium salt.[4]
Medical uses
Diclofenac is used to treat pain, inflammatory disorders, and dysmenorrhea.[9]
Pain
Inflammatory disorders may include musculoskeletal complaints, especially arthritis, rheumatoid arthritis, polymyositis, dermatomyositis, osteoarthritis, dental pain, temporomandibular joint (TMJ) pain, spondylarthritis, ankylosing spondylitis, gout attacks,[10] and pain management in cases of kidney stones and gallstones. An additional indication is the treatment of acute migraines.[11] Diclofenac is used commonly to treat mild to moderate postoperative or post-traumatic pain, in particular when inflammation is also present,[10] and is effective against menstrual pain and endometriosis.
Diclofenac is also available in topical forms and has been found to be useful for osteoarthritis but not other types of long-term musculoskeletal pain.[12]
It may also help with actinic keratosis, and acute pain caused by minor strains, sprains, and contusions (bruises).[13]
In many countries,[14] eye drops are sold to treat acute and chronic nonbacterial inflammation of the anterior part of the eyes (e.g., postoperative states). Diclofenac eye drops have also been used to manage pain for traumatic corneal abrasion.[15]
Diclofenac is often used to treat chronic pain associated with cancer, in particular if inflammation is also present (Step I of the World Health Organization (WHO) scheme for treatment of chronic pain).[16] Diclofenac can be combined with opioids if needed such as a fixed combination of diclofenac and codeine.
-
Voltaren (diclofenac) 50 mg enteric coatedtablets
-
Arthrotec (diclofenac and misoprostol) 50 mg tablets
-
Sintofarm (diclofenac) for suppositoryadministration
Contraindications
- Hypersensitivity against diclofenac
- History of allergic reactions (bronchospasm, shock, rhinitis, urticaria) following the use of other NSAIDs such as aspirin
- Third-trimester pregnancy
- Active stomach and/or duodenal ulceration or gastrointestinal bleeding
- Inflammatory bowel disease such as Crohn’s disease or ulcerative colitis
- Severe insufficiency of the heart (NYHA III/IV)
- Pain management in the setting of coronary artery bypass graft (CABG) surgery
- Severe liver insufficiency (Child-Pugh Class C)
- Severe renal insufficiency (creatinine clearance <30 ml/min)
- Caution in patients with pre-existing hepatic porphyria, as diclofenac may trigger attacks
- Caution in patients with severe, active bleeding such as cerebral hemorrhage
- NSAIDs in general should be avoided during dengue fever, as it induces (often severe) capillary leakage and subsequent heart failure.
- Caution in patients with fluid retention or heart failure
- Can lead to onset of new hypertension or worsening of pre-existing hypertension
- Can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal[17]
Adverse effects
Diclofenac consumption has been associated with significantly increased vascular and coronary risk in a study including coxib, diclofenac, ibuprofen and naproxen.[18] Upper gastrointestinal complications were also reported.[18] Major adverse cardiovascular events (MACE) were increased by about a third by diclofenac, chiefly due to an increase in major coronary events.[18] Compared with placebo, of 1000 patients allocated to diclofenac for a year, three more had major vascular events, one of which was fatal.[18] Vascular death was increased significantly by diclofenac.[18]
Heart
In 2013, a study found major vascular events were increased by about a third by diclofenac, chiefly due to an increase in major coronary events.[18] Compared with placebo, of 1000 people allocated to diclofenac for a year, three more had major vascular events, one of which was fatal.[18] Vascular death was increased by diclofenac (1·65).[18]
Following the identification of increased risks of heart attacks with the selective COX-2 inhibitor rofecoxib in 2004, attention has focused on all the other members of the NSAIDs group, including diclofenac. Research results are mixed, with a meta-analysis of papers and reports up to April 2006 suggesting a relative increased rate of heart disease of 1.63 compared to nonusers.[19] Professor Peter Weissberg, Medical Director of the British Heart Foundation said, “However, the increased risk is small, and many patients with chronic debilitating pain may well feel that this small risk is worth taking to relieve their symptoms”. Only aspirin was found not to increase the risk of heart disease; however, this is known to have a higher rate of gastric ulceration than diclofenac. In Britain the Medicines and Healthcare Products Regulatory Agency (MHRA) said in June 2013 that the drug should not be used by people with serious underlying heart conditions—people who had suffered heart failure, heart disease or a stroke were advised to stop using it completely.[20] As of January 15, 2015 the MHRA announced that diclofenac will be reclassified as a prescription-only medicine (POM) due to the risk of cardiovascular adverse events.[21]
A subsequent large study of 74,838 Danish users of NSAIDs or coxibs found no additional cardiovascular risk from diclofenac use.[22] A very large study of 1,028,437 Danish users of various NSAIDs or coxibs found the “Use of the nonselective NSAID diclofenac and the selective cyclooxygenase-2 inhibitor rofecoxib was associated with an increased risk of cardiovascular death (odds ratio, 1.91; 95% confidence interval, 1.62 to 2.42; and odds ratio, 1.66; 95% confidence interval, 1.06 to 2.59, respectively), with a dose-dependent increase in risk.”[23]
Diclofenac is similar in COX-2 selectivity to celecoxib.[24]
Gastrointestinal
- Gastrointestinal complaints are most often noted. The development of ulceration and/or bleeding requires immediate termination of treatment with diclofenac. Most patients receive a gastro-protective drug as prophylaxis during long-term treatment (misoprostol, ranitidine 150 mg at bedtime or omeprazole 20 mg at bedtime).
Liver
- Liver damage occurs infrequently, and is usually reversible. Hepatitis may occur rarely without any warning symptoms and may be fatal. Patients with osteoarthritis more often develop symptomatic liver disease than patients with rheumatoid arthritis. Liver function should be monitored regularly during long-term treatment. If used for the short-term treatment of pain or fever, diclofenac has not been found more hepatotoxic than other NSAIDs.
- As of December 2009, Endo, Novartis, and the US FDA notified healthcare professionals to add new warnings and precautions about the potential for elevation in liver function tests during treatment with all products containing diclofenac sodium.[25]
- Cases of drug-induced hepatotoxicity have been reported in the first month, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
- Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 week after initiating treatment with diclofenac.
Kidney
- NSAIDs “are associated with adverse renal [kidney] effects caused by the reduction in synthesis of renal prostaglandins“[26] in sensitive persons or animal species, and potentially during long-term use in nonsensitive persons if resistance to side effects decreases with age. However, this side effect cannot be avoided merely by using a COX-2 selective inhibitor because, “Both isoforms of COX, COX-1 and COX-2, are expressed in the kidney… Consequently, the same precautions regarding renal risk that are followed for nonselective NSAIDs should be used when selective COX-2 inhibitors are administered.”[26] However, diclofenac appears to have a different mechanism of renal toxicity.[citation needed]
- Studies in Pakistan showed diclofenac caused acute kidney failure in vultures when they ate the carcasses of animals that had recently been treated with it. Drug-sensitive species and individual humans are initially assumed to lack genes expressing specific drug detoxification enzymes.[27]
Mental health
- Mental health side effects have been reported. These symptoms are rare, but exist in significant enough numbers to include as potential side effects. These include depression, anxiety, irritability, nightmares, and psychotic reactions.[28]
Mechanism of action
The primary mechanism responsible for its anti-inflammatory, antipyretic, and analgesic action is thought to be inhibition of prostaglandin synthesis by inhibition of the transiently expressed prostaglandin-endoperoxide synthase-2 (PGES-2) also known as cycloxygenase-2 (COX-2). It also appears to exhibit bacteriostatic activity by inhibiting bacterial DNA synthesis.[29]
Inhibition of prostaglandin synthesis occurs systemically resulting in undesirable symptoms such as irritation of the gastric epithelium.[citation needed] This is the main side effect of diclofenac. Diclofenac inhibits COX-2 with 20 times greater potency than the constitutively expressed isoenzyme COX-1[30] and has, therefore, a somewhat lower incidence of gastrointestinal complaints than noted with aspirin which inhibits COX-1 to a greater extent.
The action of one single dose is much longer (6 to 8 hr) than the very short 1.2–2 hr half-life of the drug would indicate. This could be partly because it persists for over 11 hours in synovial fluids.[31]
Diclofenac may also be a unique member of the NSAIDs. Some evidence indicates it inhibits the lipoxygenase pathways, thus reducing formation of the leukotrienes(also pro-inflammatory autacoids). It also may inhibit phospholipase A2 as part of its mechanism of action. These additional actions may explain its high potency – it is the most potent NSAID on a broad basis.[32]
Marked differences exist among NSAIDs in their selective inhibition of the two subtypes of cyclooxygenase, COX-1 and COX-2. Much pharmaceutical drug design has attempted to focus on selective COX-2 inhibition as a way to minimize the gastrointestinal side effects of NSAIDs such as aspirin. In practice, use of some COX-2 inhibitors with their adverse effects has led to massive numbers of patient family lawsuits alleging wrongful death by heart attack, yet other significantly COX-selective NSAIDs, such as diclofenac, have been well tolerated by most of the population.\
Besides the COX-inhibition, a number of other molecular targets of diclofenac possibly contributing to its pain-relieving actions have recently been identified. These include:
- Blockage of voltage-dependent sodium channels (after activation of the channel, diclofenac inhibits its reactivation also known as phase inhibition)[citation needed]
- Blockage of acid-sensing ion channels (ASICs)[33]
- Positive allosteric modulation of KCNQ- and BK-potassium channels (diclofenac opens these channels, leading to hyperpolarization of the cell membrane)
Ecological effects
Use of diclofenac for animals is controversial due to toxicity when eaten by scavenging birds that eat dead animals; the drug has been banned for veterinary use in many countries.
Use of diclofenac in animals has been reported to have led to a sharp decline in the vulture population in the Indian subcontinent – a 95% decline by 2003[34] and a 99.9% decline by 2008. The mechanism is presumed to be renal failure;[35] however, toxicity may be due to direct inhibition of uric acid secretion in vultures.[36] Vultures eat the carcasses of livestockthat have been administered veterinary diclofenac, and are poisoned by the accumulated chemical,[37] as vultures do not have a particular enzyme to break down diclofenac. At a meeting of the National Wildlife Board in March 2005, the Government of India announced it intended to phase out the veterinary use of diclofenac.[38] Meloxicam is a safer alternative to replace use of diclofenac.[39] It is more expensive than diclofenac, but the price is coming down as more pharmaceutical companies begin to manufacture it.
Steppe eagles have the same vulnerability to diclofenac as vultures and may also fall victim to it.[40] Diclofenac has been shown also to harm freshwater fish species such as rainbow trout.[41][42][43][44] In contrast, New World vultures, such as the turkey vulture, can tolerate at least 100 times the level of diclofenac that is lethal to Gyps species.[45]
“The loss of tens of millions of vultures over the last decade has had major ecological consequences across the Indian Subcontinent that pose a potential threat to human health. In many places, populations of feral dogs (Canis familiaris) have increased sharply from the disappearance of Gyps vultures as the main scavenger of wild and domestic ungulatecarcasses. Associated with the rise in dog numbers is an increased risk of rabies“[39] and casualties of almost 50,000 people.[46] The Government of India cites this as one of the major consequences of a vulture species extinction.[38] A major shift in the transfer of corpse pathogens from vultures to feral dogs and rats could lead to a disease pandemic, causing millions of deaths in a crowded country like India, whereas vultures’ digestive systems safely destroy many species of such pathogens. Vultures are long-lived and slow to breed. They start breeding only at the age of six and only 50% of young survive. Even if the government ban is fully implemented, it will take several years to revive the vulture population.[47]
The loss of vultures has had a social impact on the Indian Zoroastrian Parsi community, who traditionally use vultures to dispose of human corpses in Towers of Silence, but are now compelled to seek alternative methods of disposal.[39]
Despite the vulture crisis, diclofenac remains available in other countries including many in Europe.[48] It was controversially approved for veterinary use in Spain in 2013 and continues to be available, despite Spain being home to around 90% of the European vulture population and an independent simulation showing that the drug could reduce the population of vultures by 1-8% annually. Spain’s medicine agency presented simulations suggesting that the number of deaths would be quite small.[49][50]
Formulations and trade names
The name “diclofenac” derives from its chemical name: 2-(2,6-dichloranilino) phenylacetic acid. Diclofenac was first synthesized by Alfred Sallmann and Rudolf Pfister and introduced as Voltaren by Ciba-Geigy (now Novartis) in 1973, now by Glaxo SmithKline.[51]
In the United Kingdom, United States, India, and Brazil diclofenac may be supplied as either the sodium or potassium salt; in China, it is most often supplied as the sodium salt, while in some other countries it is only available as the potassium salt.
Pennsaid is a minimally systemic prescription topical lotion formulation of 1.5% w/w diclofenac sodium, which is approved in the US, Canada and other countries for osteoarthritis of the knee.
Flector Patch, a minimally systemic topical patch formulation of diclofenac, is indicated for acute pain due to minor sprains, strains, and contusions. The patch has been approved in many other countries outside the US under different brand names.
Voltaren and Voltarol contain the sodium salt of diclofenac. In the United Kingdom, Voltarol can be supplied with either the sodium salt or the potassium salt, while Cataflam, sold in some other countries, is the potassium salt only. However, Voltarol Emulgel contains diclofenac diethylammonium, in which a 1.16% concentration is equivalent to a 1% concentration of the sodium salt. In 2016 Voltarol was one of the biggest selling branded over-the-counter medications sold in Great Britain, with sales of £39.3 million.[52]
Diclofenac is available in stomach acid-resistant formulations (25 and 50 mg), fast-disintegrating oral formulations (25 and 50 mg), powder for oral solution (50 mg), slow- and controlled-release forms (75, 100 or 150 mg), suppositories (50 and 100 mg), and injectable forms (50 and 75 mg).
Diclofenac is also available over-the-counter in some countries: 12.5 mg diclofenac as potassium salt in Switzerland (Voltaren dolo), the Netherlands (Voltaren K), and preparations containing 25 mg diclofenac as the potassium salt in Germany (various trade names), New Zealand, Australia, Japan, (Voltaren Rapid), and Sweden (Voltaren T and Diclofenac T). Diclofenac as potassium salt can be found throughout the Middle East in 25 mg and 50 mg doses (Cataflam).
Solaraze (3% diclofenac sodium gel) is topically applied, twice a day for three months, to manage the skin condition known as actinic or solar keratosis. Parazone-DP is a combination of diclofenac potassium and paracetamol, manufactured and supplied by Ozone Pharmaceuticals and Chemicals, Gujarat, India. It is sold in Uruguay alone or, in combination with orphenadrine to treat muscle spasms/pain due to injuries (Dicloflex Ion).
On 14 January 2015, diclofenac oral preparations were reclassified as prescription-only medicines in the UK. The topical preparations are still available without prescription.[53]
Diclofenac formulations are available worldwide under many different trade names.[1]
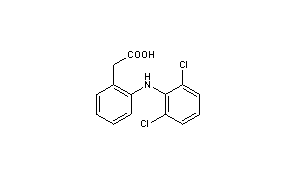 |
|
Title: Diclofenac
CAS Registry Number: 15307-86-5
CAS Name: 2-[(2,6-Dichlorophenyl)amino]benzeneacetic acid
Additional Names: [o-(2,6-dichloroanilino)phenyl]acetic acid
Trademarks: Motifene (Sankyo)
Molecular Formula: C14H11Cl2NO2
Molecular Weight: 296.15
Percent Composition: C 56.78%, H 3.74%, Cl 23.94%, N 4.73%, O 10.80%
Literature References: Prepn: NL 6604752; A. Sallmann, R. Pfister, US 3558690 (1966, 1971 both to Geigy). Pharmacology: Renaud, Lecompte, Thromb. Diath. Haemorrh. 24, 577 (1970), C.A. 74, 86215m (1971); Krupp et al., Experientia 29, 450 (1973). HPLC determn in plasma and urine: J. Godbillon et al., J. Chromatogr. 338, 151 (1985). Symposium on pharmacology and clinical experience: Semin. Arthritis Rheum. 15, Suppl. 1, 57-110 (1985); on pharmacology, efficacy and safety: Am. J. Med. 80, Suppl. 4B, 1-87 (1986). Comprehensive description: C. M. Adeyeye, P-K. Li, Anal. Profiles Drug Subs. 19, 123-144 (1990). Review of clinical trials in actinic keratosis: D. C. Peters, R. H. Foster, Drugs Aging 14, 313-319 (1999).
Properties: Crystals from ether-petr ether, mp 156-158°.
Melting point: mp 156-158°
Derivative Type: Diethylammonium salt
CAS Registry Number: 78213-16-8
Trademarks: Voltarol (Novartis)
Molecular Formula: C14H11Cl2NO2.C4H11N
Molecular Weight: 369.29
Percent Composition: C 58.54%, H 6.00%, Cl 19.20%, N 7.59%, O 8.66%
Derivative Type: Sodium salt
CAS Registry Number: 15307-79-6
Manufacturers’ Codes: GP-45840
Trademarks: Allvoran (TAD); Benfofen (Sanofi-Synthelabo); Dealgic (Pharmacia); Deflamat (Sankyo); Delphinac (Riemser); Dicloflex (Dexcel); Diclomax (Provalis); Diclophlogont (Azupharma); Dicloreum (Alfa); Duravolten (Dura); Ecofenac (Ecosol); Effekton (Teofarma); Lexobene (Merckle); Neriodin (Nagase); Novapirina (Novartis); Primofenac (Streuli); Prophenatin (Nipro); Rewodina (AWD); Rhumalgan (Sandoz); Voldal (Novartis); Voltaren (Novartis); Xenid (RPG)
Molecular Formula: C14H10Cl2NNaO2
Molecular Weight: 318.13
Percent Composition: C 52.86%, H 3.17%, Cl 22.29%, N 4.40%, Na 7.23%, O 10.06%
Properties: Crystals from water, mp 283-285°. uv max (methanol) 283 nm (e 1.05 ´ 105); (phosphate buffer, pH 7.2) 276 nm (e1.01 ´ 105). Soly at 25°C (mg/ml): deionized water (pH 5.2) >9; methanol >24; acetone 6; acetonitrile <1; cyclohexane <1; HCl (pH 1.1) <1; phosphate buffer (pH 7.2) 6. pKa 4. Partition coefficient (N-octanol/aq. buffer): 13.4. LD50 in mice, rats (mg/kg): ~390, 150 orally (Krupp).
Melting point: mp 283-285°
pKa: pKa 4
Log P: Partition coefficient (N-octanol/aq. buffer): 13.4
Absorption maximum: uv max (methanol) 283 nm (e 1.05 ´ 105); (phosphate buffer, pH 7.2) 276 nm (e 1.01 ´ 105)
Toxicity data: LD50 in mice, rats (mg/kg): ~390, 150 orally (Krupp)
Derivative Type: Potassium salt
CAS Registry Number: 15307-81-0
Manufacturers’ Codes: CGP-45840B
Trademarks: Cataflam (Novartis)
Molecular Formula: C14H10Cl2KNO2
Molecular Weight: 334.24
Percent Composition: C 50.31%, H 3.02%, Cl 21.21%, K 11.70%, N 4.19%, O 9.57%
Therap-Cat: Anti-inflammatory.
Keywords: Anti-inflammatory (Nonsteroidal); Arylacetic Acid Derivatives.
|
Synthesis


The mechanism begins with the condensation of hydrazine onto a ketone (details not shown) to give a hydrazone. Under basic conditions, this hydrazone is deprotonated at nitrogen to give an anionic intermediate. In this case, the negative charge can be delocalized onto oxygen, resulting in an enolate structure. Typically, the negative charge is only shared between a nitrogen and carbon, so this substrate gives a particularly stable intermediate. Protonation of the enolate at carbon gives the first C-H bond necessary to form the product. A second deprotonation at nitrogen gives a similar flow of electrons to form another enolate structure, this time with cleavage of the C-N bond and release of nitrogen gas. Another C-protonation gives the lactam precursor to diclofenac. Cleavage of the amide with hydroxide (details not shown) gives the target.
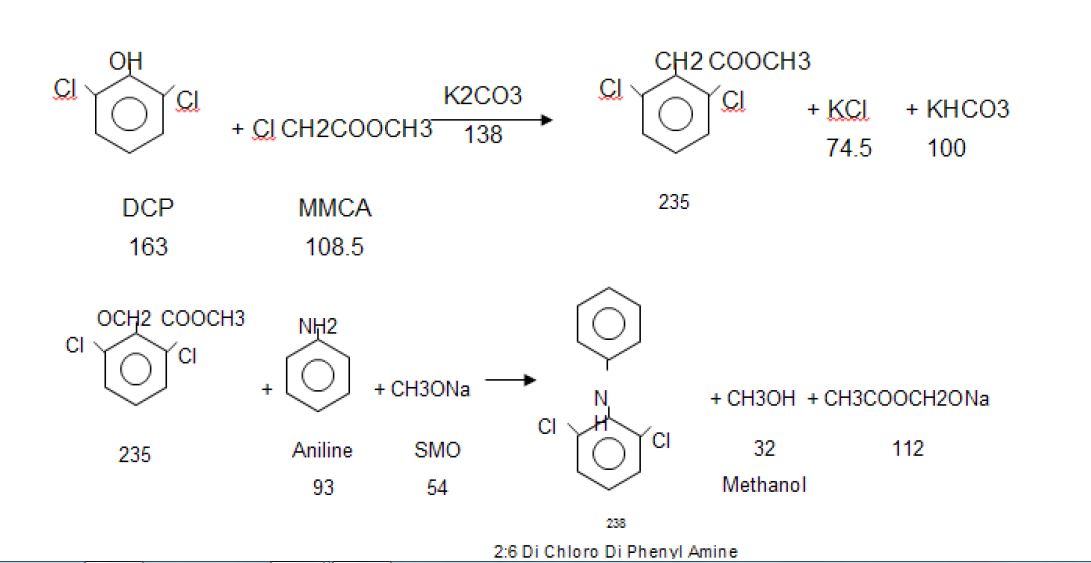
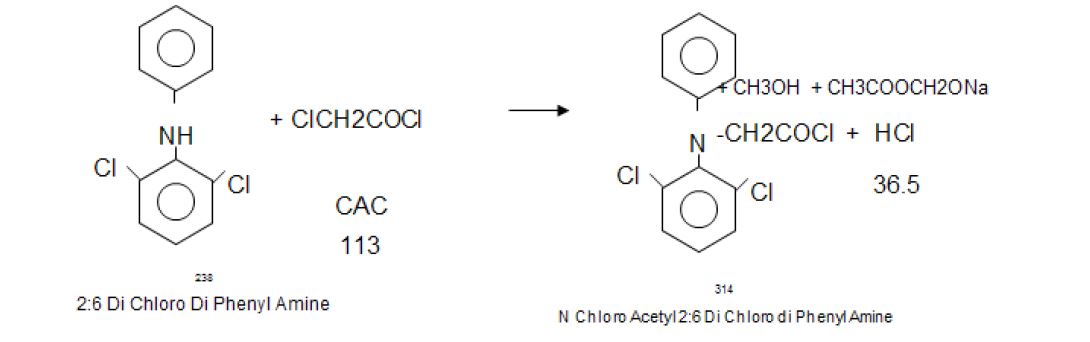
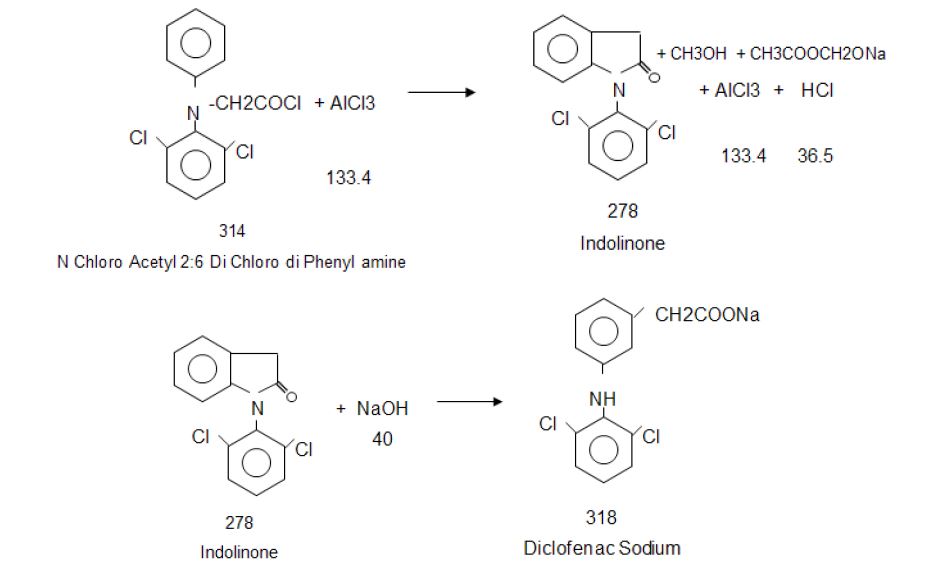
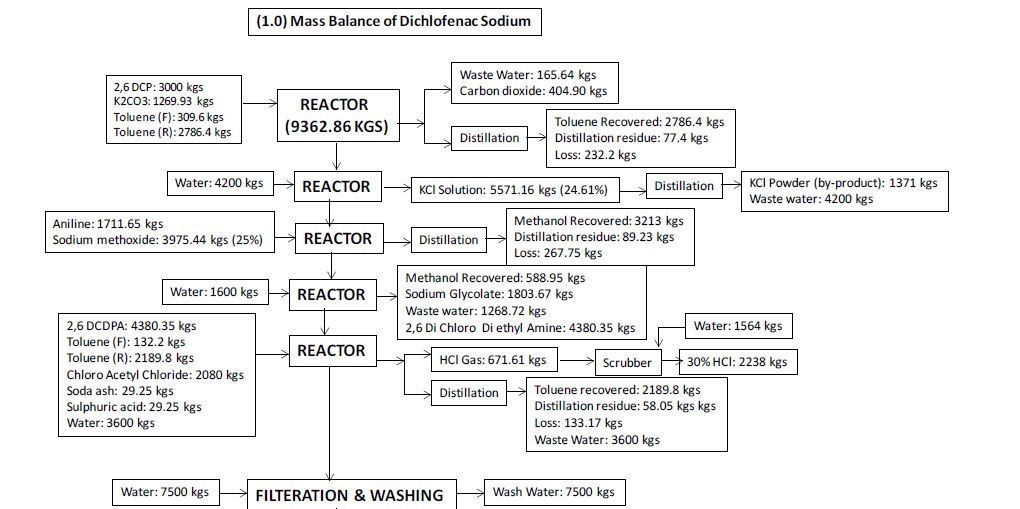
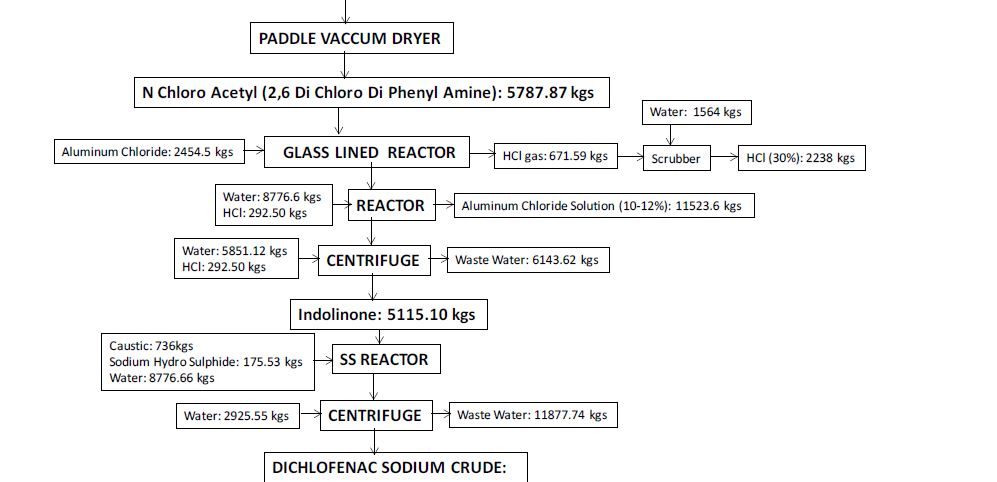
Manufacturing Process
2, 6-Dichlorophenol is reacted with MMCA, Aniline and Chloro Acetyl Chloride and AlCl3 to yield (2, 6 –
Dichlorophenol) Indolinone is hydrolyzed using isopropyl alcohol and sodium hydroxide to give crude Diclofenac
Sodium. This on purification using deminerlised water and isopropyl alcohol gives the pure Diclofenac Sodium
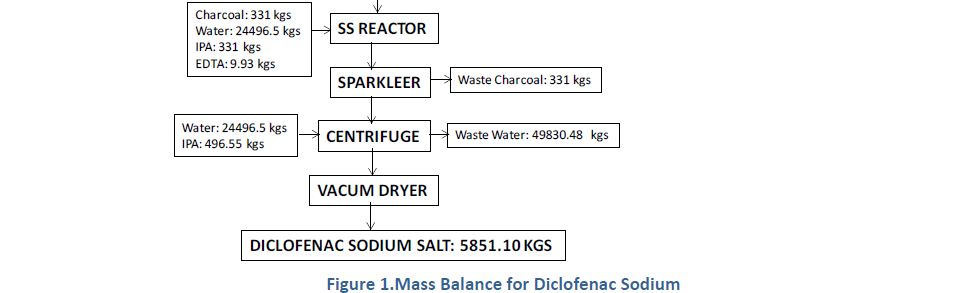
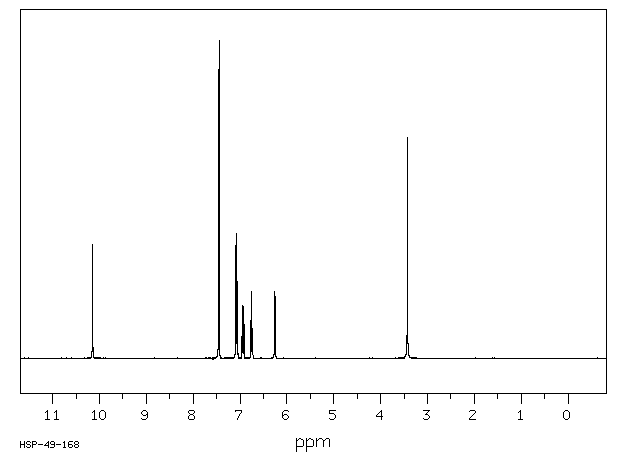
CLIP
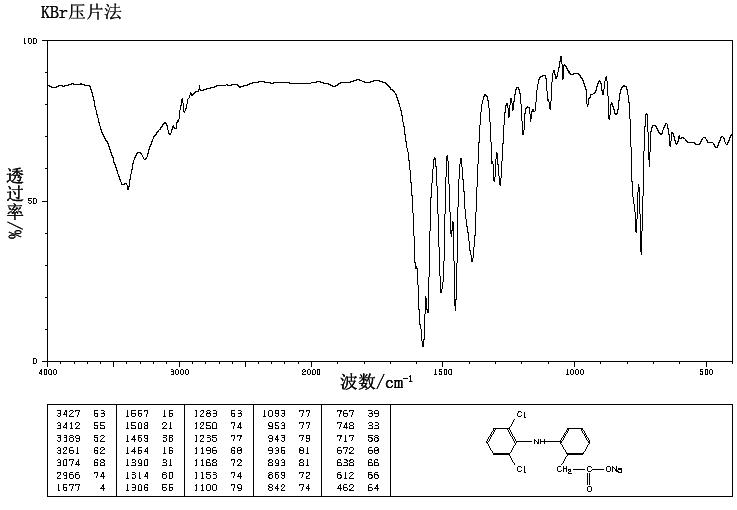
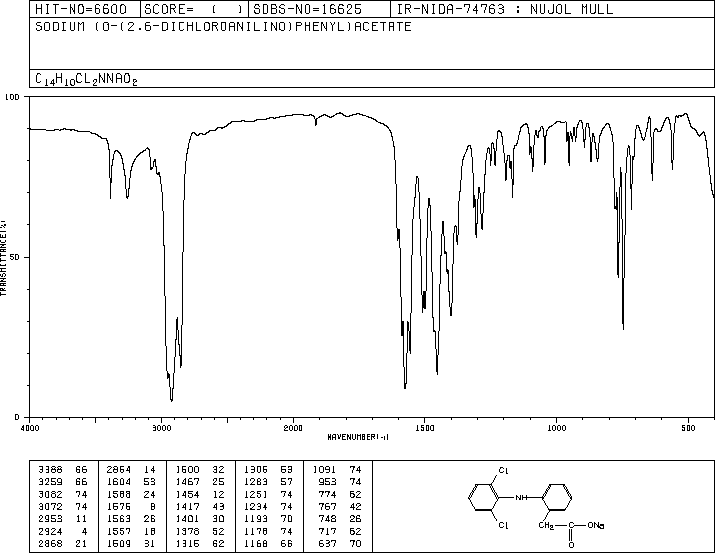

References
- ^ Jump up to:a b “Diclofenac”. Drugs.com. Retrieved 22 December 2018.
- ^ Mujib sayyad (August 23, 2018). “Diclofenac Oral Uses, Dosage, Side Effects And Composition”. Medicine Reviews Agency.
- ^ Jump up to:a b c d e f g h i “Diclofenac epolamine Monograph for Professionals”. Drugs.com. AHFS. Retrieved 22 December 2018.
- ^ Jump up to:a b c d British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. pp. 1033–1035. ISBN 978-0857112989.
- ^ Mosby’s Drug Reference for Health Professions. Elsevier Health Sciences. 2017. p. 398. ISBN 9780323566827.
- ^ Fischer, Janos (2006). Analogue-based drug discovery. Wiley-VCH. p. 517. ISBN 3527312579.
- ^ “NADAC as of 2018-12-19”. Centers for Medicare and Medicaid Services. Retrieved 22 December 2018.
- ^ “The Top 300 of 2019”. clincalc.com. Retrieved 22 December 2018.
- ^ “Diclofenac Epolamine”. The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ Jump up to:a b “RUFENAL”. Birzeit Pharmaceutical Company. Archived from the original on 2011-05-26.
- ^ “Patient Site – CAMBIA (diclofenac potassium) for oral solution”. cambiarx.
- ^ Dutta, NK; Mazumdar, K; Dastidar, SG; Park, JH (October 2007). “Activity of diclofenac used alone and in combination with streptomycin against Mycobacterium tuberculosis in mice”. International journal of antimicrobial agents. 30 (4): 336–40. doi:10.1016/j.ijantimicag.2007.04.016. PMID 17644321.
- ^ “Diclofenac (Topical Application Route) Description and Brand Names – Mayo Clinic”. www.mayoclinic.com.
- ^ cbg-meb.nl, SPC Netherlands
- ^ Wakai A, Lawrenson JG, Lawrenson AL, Wang Y, Brown MD, Quirke M, Ghandour O, McCormick R, Walsh CD, Amayem A, Lang E, Harrison N (2017). “Topical non-steroidal anti-inflammatory drugs for analgesia in traumatic corneal abrasions”. Cochrane Database Syst Rev. 5: CD009781. doi:10.1002/14651858.CD009781.pub2. PMID 28516471.
- ^ “WHO – WHO’s cancer pain ladder for adults”. www.who.int.
- ^ “Diclofenac Potassium”. Drugs.com. Drugsite Trust. Retrieved 2015-11-15.
- ^ Jump up to:a b c d e f g h Bhala, N.; Emberson, J.; et al. (2013). “Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials”. The Lancet. 382 (9894): 769–779. doi:10.1016/S0140-6736(13)60900-9. PMC 3778977. PMID 23726390.
- ^ Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C (2006). “Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials”. BMJ. 332(7553): 1302–8. doi:10.1136/bmj.332.7553.1302. PMC 1473048. PMID 16740558.
- ^ “Heart risk warning over painkiller”. 29 June 2013 – via www.bbc.co.uk.
- ^ “Press release: Diclofenac tablets now only available as a prescription medicine”. Medicines and Healthcare Products Regulatory Agency. January 14, 2015. Retrieved January 14, 2015.
- ^ Solomon DH, Avorn J, Stürmer T, Glynn RJ, Mogun H, Schneeweiss S (2006). “Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk”. Arthritis Rheum. 54 (5): 1378–89. doi:10.1002/art.21887. PMID 16645966.
- ^ Fosbøl EL, Folke F, Jacobsen S, Rasmussen JN, Sørensen R, Schramm TK, Andersen SS, Rasmussen S, Poulsen HE, Køber L, Torp-Pedersen C, Gislason GH (2010). “Cause-Specific Cardiovascular Risk Associated With Nonsteroidal Antiinflammatory Drugs Among Healthy Individuals”. Circ Cardiovasc Qual Outcomes. 3 (4): 395–405. doi:10.1161/CIRCOUTCOMES.109.861104. PMID 20530789.
- ^ FitzGerald GA, Patrono C (2001). “The coxibs, selective inhibitors of cyclooxygenase-2”. N Engl J Med. 345 (6): 433–42. doi:10.1056/NEJM200108093450607. PMID 11496855.
- ^ “fda.gov”.
- ^ Jump up to:a b Brater DC (2002). “Renal effects of cyclooxygyenase-2-selective inhibitors”. J Pain Symptom Manage. 23 (4 Suppl): S15–20, discussion S21–3. doi:10.1016/S0885-3924(02)00370-6. PMID 11992745.
- ^ Becker, Rachel. “Cattle drug threatens thousands of vultures”. Nature. doi:10.1038/nature.2016.19839.
- ^ “Diclofenac Side Effects”. Drugs.com. Retrieved 21 January 2013.
- ^ Dastidar SG, Ganguly K, Chaudhuri K, Chakrabarty AN (2000). “The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis”. Int. J. Antimicrob. Agents. 14 (3): 249–51. doi:10.1016/S0924-8579(99)00159-4. PMID 10773497.
- ^ Cryer, B.; Feldman, M. (1998). “Cyclooxygenase-1 and Cyclooxygenase-2 Selectivity of Widely Used Nonsteroidal Anti-Inflammatory Drugs”. The American Journal of Medicine. 104(5): 413–421. doi:10.1016/S0002-9343(98)00091-6.
- ^ Fowler PD, Shadforth MF, Crook PR, John VA (1983). “Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis”. Eur. J. Clin. Pharmacol. 25 (3): 389–94. doi:10.1007/BF01037953. PMID 6628528.
- ^ Scholer. Pharmacology of Diclofenac Sodium. Am J of Medicine Volume 80 April 28, 1986
- ^ Voilley N, de Weille J, Mamet J, Lazdunski M: Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001 Oct 15;21(20):8026-33.
- ^ Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, Shivaprasad HL, Ahmed S, Chaudhry MJ, Arshad M, Mahmood S, Ali A, Khan AA (2004). “Diclofenac residues as the cause of vulture population decline in Pakistan”. Nature. 427 (6975): 630–3. Bibcode:2004Natur.427..630O. doi:10.1038/nature02317. PMID 14745453.
- ^ Swan, Gerry E.; Cuthbert, Richard; Quevedo, Miguel; Green, Rhys E.; Pain, Deborah J.; Bartels, Paul; Cunningham, Andrew A.; Duncan, Neil; Meharg, Andrew A.; Oaks, J. Lindsay; Parry-Jones, Jemima; Shultz, Susanne; Taggart, Mark A.; Verdoorn, Gerhard; Wolter, Kerri (2006-06-22). “Toxicity of diclofenac to Gyps vultures”. Biology Letters. 2 (2): 279–282. doi:10.1098/rsbl.2005.0425. ISSN 1744-9561. PMC 1618889. PMID 17148382.
- ^ Naidoo V, Swan GE (August 2008). “Diclofenac toxicity in Gyps vulture is associated with decreased uric acid excretion and not renal portal vasoconstriction”. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 149 (3): 269–74. doi:10.1016/j.cbpc.2008.07.014. PMID 18727958.
- ^ “Vet drug ‘killing Asian vultures‘“. BBC News. 2004-02-28.
- ^ Jump up to:a b “Saving the Vultures from Extinction” (Press release). Press Information Bureau, Government of India. 2005-05-16. Retrieved 2006-05-12.
- ^ Jump up to:a b c Swan G, Naidoo V, Cuthbert R, Green RE, Pain DJ, Swarup D, Prakash V, Taggart M, Bekker L, Das D, Diekmann J, Diekmann M, Killian E, Meharg A, Patra RC, Saini M, Wolter K (2006). “Removing the threat of diclofenac to critically endangered Asian vultures”. PLoS Biol. 4 (3): e66. doi:10.1371/journal.pbio.0040066. PMC 1351921. PMID 16435886.
- ^ Phadnis, Mayuri (May 28, 2014). “Eagles fall prey to vulture-killing chemical”. Pune Mirror. Retrieved May 28, 2014.
- ^ Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD (2004). “Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: Histopathological alterations and bioaccumulation in rainbow trout”. Aquat. Toxicol. 68 (2): 141–150. doi:10.1016/j.aquatox.2004.03.014. PMID 15145224.
- ^ Triebskorn R, Casper H, Heyd A, Eikemper R, Köhler HR, Schwaiger J (2004). “Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part II: Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss)”. Aquat. Toxicol. 68(2): 151–166. doi:10.1016/j.aquatox.2004.03.015. PMID 15145225.
- ^ Schwaiger & Triebskorn (2005). UBA-Berichte 29/05: 217-226.
- ^ Triebskorn R, Casper H, Scheil V, Schwaiger J (2007). “Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio)”. Anal Bioanal Chem. 387 (4): 1405–16. doi:10.1007/s00216-006-1033-x. PMID 17216161.
- ^ Rattner BA, Whitehead MA, Gasper G, Meteyer CU, Link WA, Taggart MA, Meharg AA, Pattee OH, Pain DJ (2009). “Apparent tolerance of turkey vultures (Cathartes aura) to the non-steroidal anti-inflammatory drug diclofenac”. Environmental Toxicology and Chemistry. 27 (11): 2341–2345. doi:10.1897/08-123.1. PMID 18476752.
- ^ Walker, Matt (August 6, 2008). “Rabies tragedy follows loss of India’s vultures”. New Scientist.
- ^ Choudhary, Srishti (August 29, 2016). “‘Decline in vulture population has given rise to diseases’: Dr Vibhu Prakash”. The Indian Express. Retrieved December 12, 2018.
- ^ “E-010588/2015: answer given by Mr Andriukaitis on behalf of the Commission”. European Parliament. Retrieved 2 May 2016.
- ^ Becker, Rachel. “Cattle drug threatens thousands of vultures”. Nature. Retrieved 2 May2016.
- ^ International, BirdLife. “Vulture killing drug now available on EU market”. www.birdlife.org.
- ^ Altman, R; Bosch, B; Brune, K; Patrignani, P; Young, C (May 2015). “Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology”. Drugs. 75(8): 859–77. doi:10.1007/s40265-015-0392-z. PMID 25963327.
- ^ “A breakdown of the over-the-counter medicines market in Britain in 2016”. Pharmaceutical Journal. 28 April 2017. Retrieved 29 May 2017.
- ^ “Oral diclofenac presentations with legal status ‘P’ – reclassified to POM – GOV.UK”. www.gov.uk.
External links
References
-
- US 3 558 690 (Geigy; 26.1.1971; CH-prior. 8.4.1965, 25.2.1966, 30.3.1966, 20.12.1967).
- DAS 1 543 639 (Ciba-Geigy; appl. 7.4.1966; CH-prior. 8.4.1965).
- DAS 1 793 592 (Ciba-Geigy; appl. 7.4.1966; CH-prior. 8.4.1965).
- US 3 652 762 (Ciba-Geigy; 28.3.1972; prior. 9.12.1968, 29.9.1969, 14.4.1970).
- US 3 778 470 (Geigy; 11.12.1973; appl. 2.10.1970; prior. 4.4.1966).
- CH 492 679 (Geigy; appl. 30.3.1966).
-
Alternative synthesis:
- DOS 2 613 838 (Ikeda Mohando; appl. 31.3.1976; J-prior. 31.3.1975).
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Cataflam, Voltaren, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689002 |
| Pregnancy category |
|
| Routes of administration |
By mouth, rectal, intramuscular, intravenous(renal- and gallstones), topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | More than 99% |
| Metabolism | Liver, oxidative, primarily by CYP2C9, also by CYP2C8, CYP3A4, as well as conjugative by glucuronidation (UGT2B7) and sulfation;[2] no active metabolites exist |
| Elimination half-life | 1.2–2 hr (35% of the drug enters enterohepatic recirculation) |
| Excretion | 40% biliary 60% urine |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.035.755 |
| Chemical and physical data | |
| Formula | C14H11Cl2NO2 |
| Molar mass | 296.148 g/mol |
| 3D model (JSmol) | |
Diclofenac
- ATC:M01AB05; M02AA15; S01BC03
- MW:296.15 g/mol
- CAS-RN:15307-86-5
- InChI Key:DCOPUUMXTXDBNB-UHFFFAOYSA-N
- InChI:InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)
- EINECS:239-348-5
- LD50:170 mg/kg (M, p.o.);
62.5 mg/kg (R, p.o.)
Monosodium salt
- Formula:C14H10Cl2NNaO2
- MW:318.14 g/mol
- CAS-RN:15307-79-6
- EINECS:239-346-4
- LD50:116 mg/kg (M, i.v.); 390 mg/kg (M, p.o.);
117 mg/kg (R, i.v.); 150 mg/kg (R, p.o.)
//////////////Diclofenac Sodium
C1=CC=C(C(=C1)CC(=O)[O-])NC2=C(C=CC=C2Cl)Cl.[Na+]
Diclofenac Sodium
| FDA Orange Book Patents: 1 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9339551 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 2 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9339552 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 3 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9415029 |
| Expiration | Jul 10, 2029 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 4 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9370501 |
| Expiration | Jul 10, 2029 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 5 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9375412 |
| Expiration | Jul 10, 2029 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 6 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8946292 |
| Expiration | Mar 22, 2027 |
| Applicant | JAVELIN PHARMS INC |
| Drug Application | N022396 (Prescription Drug: DYLOJECT. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 7 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9168305 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 8 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9168304 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 9 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9220784 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 10 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 6407079 |
| Expiration | Jun 18, 2019 |
| Applicant | JAVELIN PHARMS INC |
| Drug Application | N022396 (Prescription Drug: DYLOJECT. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 11 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8252838 |
| Expiration | Apr 21, 2028 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 12 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8618164 |
| Expiration | Jul 10, 2029 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 13 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8546450 |
| Expiration | Aug 9, 2030 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 14 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8217078 |
| Expiration | Jul 10, 2029 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 15 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8563613 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 16 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8871809 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 17 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9066913 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 18 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 8741956 |
| Expiration | Jul 10, 2029 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |
| FDA Orange Book Patents: 19 of 21 (FDA Orange Book Patent ID) | |
|---|---|
| Patent | 9101591 |
| Expiration | Oct 17, 2027 |
| Applicant | HZNP |
| Drug Application | N204623 (Prescription Drug: PENNSAID. Ingredients: DICLOFENAC SODIUM) |