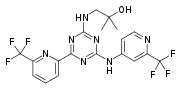
Enasidenib
- Molecular Formula C19H17F6N7O
- Average mass 473.375
- 2-Methyl-1-[[4-[6-(trifluoromethyl)-2-pyridinyl]-6-[[2-(trifluoromethyl)-4-pyridinyl]amino]-1,3,5-triazin-2-yl]amino]-2-propanol
- 2-Methyl-1-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)pyridin-4-ylamino)-1,3,5-triazin-2-ylamino)propan-2-ol
| Originator | Agios Pharmaceuticals |
|---|---|
| Developer | Celgene Corporation |
| Mechanism Of Action | Isocitrate dehydrogenase 2 inhibitor |
| Who Atc Codes | L01 (Antineoplastic Agents) |
| Ephmra Codes | L1 (Antineoplastics) |
| Indication | Cancer |
Enasidenib mesylate [USAN]
RN: 1650550-25-6
UNII: UF6PC17XAV
Molecular Formula, C19-H17-F6-N7-O.C-H4-O3-S
Molecular Weight, 569.4849
2-Propanol, 2-methyl-1-((4-(6-(trifluoromethyl)-2-pyridinyl)-6-((2-(trifluoromethyl)-4-pyridinyl)amino)-1,3,5-triazin-2-yl)amino)-, methanesulfonate (1:1)
Enasidenib (AG-221) is an experimental drug in development for treatment of cancer. It is a small molecule inhibitor of IDH2 (isocitrate dehydrogenase 2). It was developed by Agios Pharmaceuticals and is licensed to Celgene for further development.

LC MS
https://file.medchemexpress.com/batch_PDF/HY-18690/Enasidenib_LCMS_18195_MedChemExpress.pdf
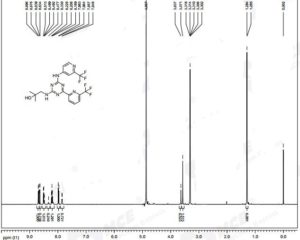
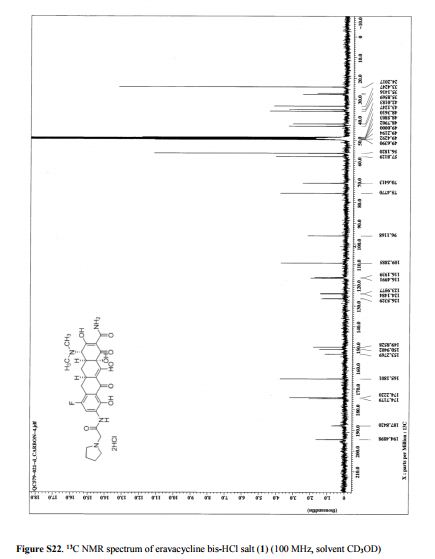
NMR FROM INTERNET SOURCES
SEE http://www.medkoo.com/uploads/product/Enasidenib__AG-221_/qc/QC-Enasidenib-TZC60322Web.pdf
see also
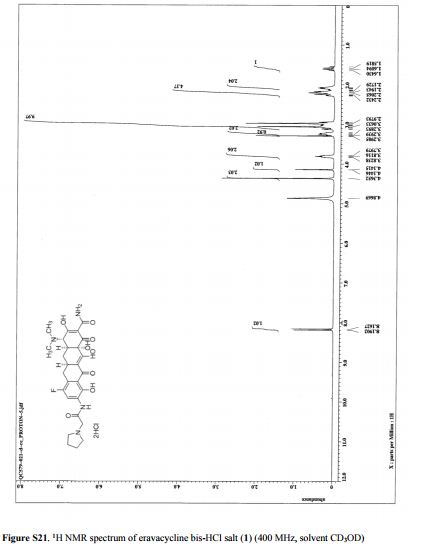
https://file.medchemexpress.com/batch_PDF/HY-18690/Enasidenib_HNMR_18195_MedChemExpress.pdf ……….NMR CD3OD
Patent
http://www.google.com/patents/US20130190287
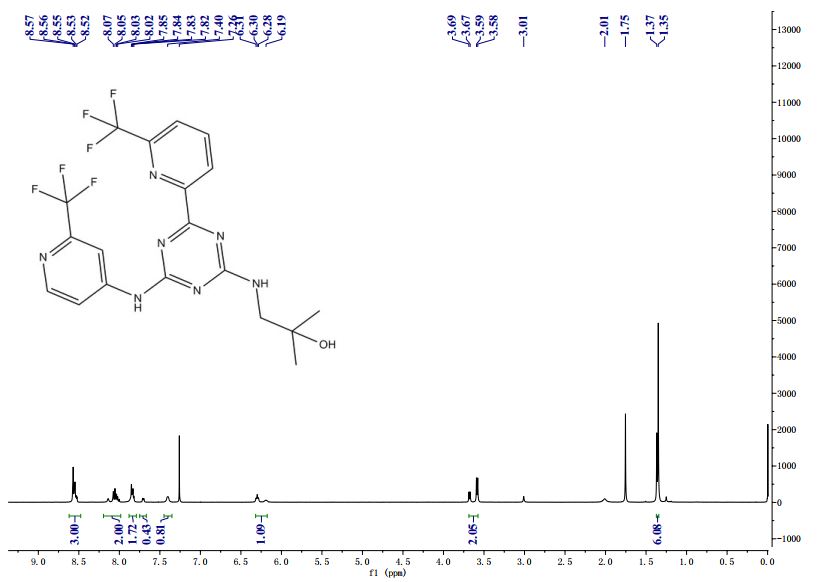
Compound 409—2-methyl-1-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)pyridin-4-ylamino)-1,3,5-triazin-2-ylamino)propan-2-ol
1H NMR (METHANOL-d4) δ 8.62-8.68 (m, 2H), 847-8.50 (m, 1H), 8.18-8.21 (m, 1H), 7.96-7.98 (m, 1H), 7.82-7.84 (m, 1H), 3.56-3.63 (d, J=28 Hz, 2H), 1.30 (s, 6H). LC-MS: m/z 474.3 (M+H)+.
‘The FDA granted fast track designation and orphan drug status for acute myeloid leukemia in 2014.[1]
An orally available inhibitor of isocitrate dehydrogenase type 2 (IDH2), with potential antineoplastic activity. Upon administration, AG-221 specifically inhibits IDH2 in the mitochondria, which inhibits the formation of 2-hydroxyglutarate (2HG). This may lead to both an induction of cellular differentiation and an inhibition of cellular proliferation in IDH2-expressing tumor cells. IDH2, an enzyme in the citric acid cycle, is mutated in a variety of cancers; It initiates and drives cancer growth by blocking differentiation and the production of the oncometabolite 2HG.
Isocitrate dehydrogenases (IDHs) catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate (i.e., a-ketoglutarate). These enzymes belong to two distinct subclasses, one of which utilizes NAD(+) as the electron acceptor and the other NADP(+). Five isocitrate dehydrogenases have been reported: three NAD(+)-dependent isocitrate dehydrogenases, which localize to the mitochondrial matrix, and two NADP(+)-dependent isocitrate dehydrogenases, one of which is mitochondrial and the other predominantly cytosolic. Each NADP(+)-dependent isozyme is a homodimer.
IDH2 (isocitrate dehydrogenase 2 (NADP+), mitochondrial) is also known as IDH; IDP; IDHM; IDPM; ICD-M; or mNADP-IDH. The protein encoded by this gene is the
NADP(+)-dependent isocitrate dehydrogenase found in the mitochondria. It plays a role in intermediary metabolism and energy production. This protein may tightly associate or interact with the pyruvate dehydrogenase complex. Human IDH2 gene encodes a protein of 452 amino acids. The nucleotide and amino acid sequences for IDH2 can be found as GenBank entries NM_002168.2 and NP_002159.2 respectively. The nucleotide and amino acid sequence for human IDH2 are also described in, e.g., Huh et al., Submitted (NOV-1992) to the
EMBL/GenBank/DDBJ databases; and The MGC Project Team, Genome Res.
14:2121-2127(2004).
Non-mutant, e.g., wild type, IDH2 catalyzes the oxidative decarboxylation of isocitrate to a-ketoglutarate (a- KG) thereby reducing NAD+ (NADP+) to NADH (NADPH), e.g., in the forward reaction:
Isocitrate + NAD+ (NADP+)→ a-KG + C02 + NADH (NADPH) + H+.
It has been discovered that mutations of IDH2 present in certain cancer cells result in a new ability of the enzyme to catalyze the NAPH-dependent reduction of α-ketoglutarate to R(-)-2-hydroxyglutarate (2HG). 2HG is not formed by wild- type IDH2. The production of 2HG is believed to contribute to the formation and progression of cancer (Dang, L et al, Nature 2009, 462:739-44).
The inhibition of mutant IDH2 and its neoactivity is therefore a potential therapeutic treatment for cancer. Accordingly, there is an ongoing need for inhibitors of IDH2 mutants having alpha hydroxyl neoactivity.
Mechanism of action
Isocitrate dehydrogenase is a critical enzyme in the citric acid cycle. Mutated forms of IDH produce high levels of 2-hydroxyglutarate and can contribute to the growth of tumors. IDH1 catalyzes this reaction in the cytoplasm, while IDH2 catalyzes this reaction in mitochondria. Enasidenib disrupts this cycle.[1][2]
Development
The drug was discovered in 2009, and an investigational new drug application was filed in 2013. In an SEC filing, Agios announced that they and Celgene were in the process of filing a new drug application with the FDA.[3] The fast track designation allows this drug to be developed in what in markedly less than the average 14 years it takes for a drug to be developed and approved.[4]
PATENT
Compound 409 –
2-methyl-l-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)pyri^
ίαζίη-2- lamino ropan-2-ol
1H NMR (METHANOL-d4) δ 8.62-8.68 (m, 2 H), 847-8.50 (m, 1 H), 8.18-8.21 (m, 1 H), 7.96-7.98 (m, 1 H), 7.82-7.84 (m, 1 H), 3.56-3.63 (d, J = 28 Hz, 2 H), 1.30 (s, 6 H). LC-MS: m/z 474.3 (M+H)+.
WO 2017066611
WO 2017024134
WO 2016177347
PATENT
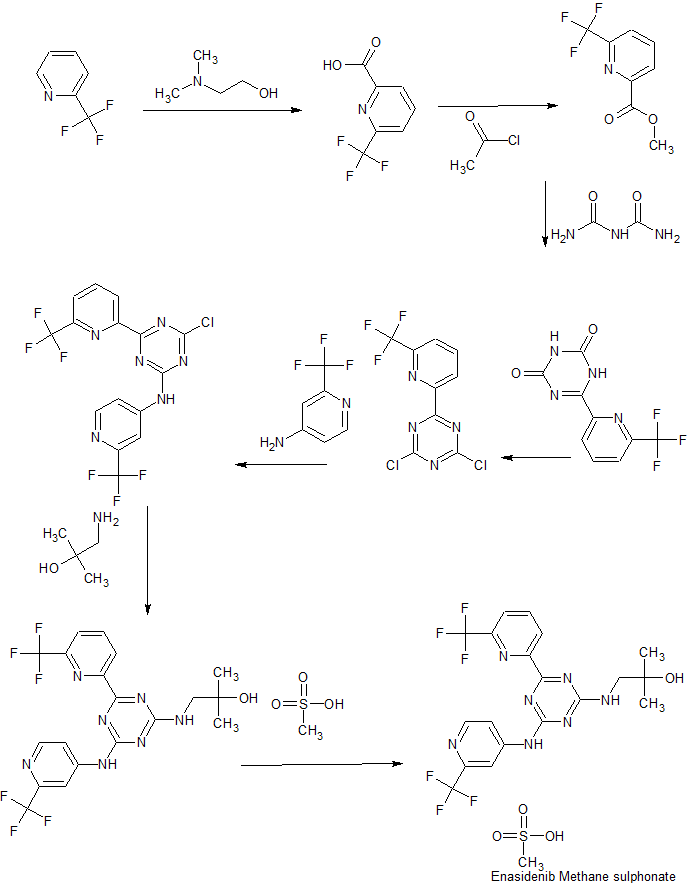
Example 1: Synthesis of compound 3
Example 1, Step 1: preparation of 6-trifluoromethyl-pyridine-2-carboxylic acid
Diethyl ether (4.32 L) and hexanes (5.40 L) are added to the reaction vessel under N2 atmosphere, and cooled to -75 °C to -65 °C. Dropwise addition of n-Butyl lithium (3.78 L in 1.6 M hexane) under N2 atmosphere at below -65 °C is followed by dropwise addition of dimethyl amino ethanol (327.45 g, 3.67 mol) and after 10 min. dropwise addition of 2-trifluoromethyl pyridine (360 g, 2.45 mol). The reaction is stirred under N2 while maintaining the temperature below -65 °C for about 2.0-2.5 hrs. The reaction mixture is poured over crushed dry ice under N2, then brought to a temperature of 0 to 5 °C while stirring (approx. 1.0 to 1.5 h) followed by the addition of water (1.8 L). The reaction mixture is stirred for 5-10 mins and allowed to warm to 5-10 °C. 6N HC1 (900 mL) is added dropwise until the mixture reached pH 1.0 to 2.0, then the mixture is stirred for 10-20 min. at 5-10 °C. The reaction mixture is diluted with ethyl acetate at 25-35 °C, then washed with brine solution. The reaction is concentrated and rinsed with n-heptane and then dried to yield 6-trifluoromethyl-pyridine-2-carboxylic acid.
Example 1, Step 2: preparation of 6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester Methanol is added to the reaction vessel under nitrogen atmosphere. 6-trifluoromethyl- pyridine-2-carboxylic acid (150 g, 0.785 mol) is added and dissolved at ambient temperature. Acetyl chloride (67.78 g, 0.863 mol) is added dropwise at a temperature below 45 °C. The reaction mixture is maintained at 65-70 °C for about 2-2.5 h, and then concentrated at 35-45 °C under vacuum and cooled to 25-35 °C. The mixture is diluted with ethyl acetate and rinsed with saturated NaHC03 solution then rinsed with brine solution. The mixture is concentrated at temp 35-45 °C under vacuum and cooled to 25-35 °C, then rinsed with n-heptane and concentrated at temp 35-45 °C under vacuum, then degassed to obtain brown solid, which is rinsed with n-heptane and stirred for 10-15 minute at 25-35 °C. The suspension is cooled to -40 to -30 °C while stirring, and filtered and dried to provide 6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester.
Example 1, Step 3: preparation of 6-(6-Trifluoromethyl-pyridin-2-yl)-lH-l,3,5-triazine-2,4-dione
1 L absolute ethanol is charged to the reaction vessel under N2 atmosphere and Sodium Metal (11.2 g, 0.488 mol) is added in portions under N2 atmosphere at below 50 °C. The reaction is stirred for 5-10 minutes, then heated to 50-55 °C. Dried Biuret (12.5 g, 0.122 mol) is added to the reaction vessel under N2 atmosphere at 50-55 °C temperature, and stirred 10-15 minutes. While maintaining 50-55 °C 6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester (50.0 g, 0.244 mol) is added. The reaction mixture is heated to reflux (75-80 °C) and maintained for 1.5-2 hours. Then cooled to 35-40 °C, and concentrated at 45-50 °C under vacuum. Water is added and the mixture is concentrated under vacuum then cooled to 35-40 °C more water is added and the mixture cooled to 0 -5 °C. pH is adjusted to 7-8 by slow addition of 6N HC1, and solid precipitated out and is centrifuged and rinsed with water and centrifuged again. The off white to light brown solid of 6-(6-Trifluoromethyl-pyridin-2-yl)-lH-l,3,5-triazine-2,4-dione is dried under vacuum for 8 to 10 hrs at 50 °C to 60 °C under 600mm/Hg pressure to provide 6-(6-Trifluoromethyl-pyridin-2-yl)-lH-l,3,5-triazine-2,4-dione.
Example 1, Step 4: preparation of 2, 4-Dichloro-6-(6-trifluoromethyl-pyridin-2-yl)-l, 3, 5-triazine
POCI3 (175.0 mL) is charged into the reaction vessel at 20- 35 °C, and 6-(6-Trifluoromethyl-pyridin-2-yl)-lH-l,3,5-triazine-2,4-dione (35.0 g, 0.1355 mol) is added in portions at below 50 °C. The reaction mixture is de-gassed 5-20 minutes by purging with N2 gas. Phosphorous pentachloride (112.86 g, 0.542 mol) is added while stirring at below 50 °C and the resulting slurry is heated to reflux (105-110 °C) and maintained for 3-4 h. The reaction mixture is cooled to 50-55 °C, and concentrated at below 55 °C then cooled to 20-30 °C. The reaction mixture is rinsed with ethyl acetate and the ethyl acetate layer is slowly added to cold water (temperature ~5 °C) while stirring and maintaining the temperature below 10 °C. The mixture is stirred 3-5 minutes at a temperature of between 10 to 20 °C and the ethyl acetate layer is collected. The reaction mixture is rinsed with sodium bicarbonate solution and dried over anhydrous sodium sulphate. The material is dried 2-3 h under vacuum at below 45 °C to provide 2, 4-Dichloro-6-(6-trifluoromethyl-pyridin-2-yl)-l, 3, 5-triazine. Example 1, Step 5: preparation of 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)- pyridin-4-yl)-l,3,5-triazin-2-amine
A mixture of THF (135 mL) and 2, 4-Dichloro-6-(6-trifluoromethyl-pyridin-2-yl)-l, 3, 5-triazine (27.0 g, 0.0915 mol) are added to the reaction vessel at 20 – 35 °C, then 4-amino-2-(trifluoromethyl)pyridine (16.31 g, 0.1006 mol) and sodium bicarbonate (11.52 g, 0.1372 mol) are added. The resulting slurry is heated to reflux (75-80 °C) for 20-24 h. The reaction is cooled to 30-40 °C and THF evaporated at below 45 °C under reduced pressure. The reaction mixture is cooled to 20-35 °C and rinsed with ethyl acetate and water, and the ethyl acetate layer collected and rinsed with 0.5 N HC1 and brine solution. The organic layer is concentrated under vacuum at below 45 °C then rinsed with dichloromethane and hexanes, filtered and washed with hexanes and dried for 5-6h at 45-50 °C under vacuum to provide 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)- pyridin-4-yl)-l,3,5-triazin-2-amine.
Example 1, Step 6: preparation of 2-methyl-l-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)- pyridin-4-ylamino)-l,3,5-triazin-2-ylamino)propan-2-ol
THF (290 mL), 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)-pyridin-4-yl)-l,3,5-triazin-2-amine (29.0 g, 0.06893 mol), sodium bicarbonate (8.68 g, 0.1033 mol), and 1, 1-dimethylaminoethanol (7.37 g, 0.08271 mol) are added to the reaction vessel at 20-35 °C. The resulting slurry is heated to reflux (75-80 °C) for 16-20 h. The reaction is cooled to 30-40 °C and THF evaporated at below 45 °C under reduced pressure. The reaction mixture is cooled to 20-35 °C and rinsed with ethyl acetate and water, and the ethyl acetate layer collected. The organic layer is concentrated under vacuum at below 45 °C then rinsed with dichlorom ethane and hexanes, filtered and washed with hexanes and dried for 8-1 Oh at 45-50 °C under vacuum to provide 2-methyl-l-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)- pyridin-4-ylamino)-l,3,5-triazin-2-ylamino)propan-2-ol.
PATENT
US 20160089374
PATENT
WO 2015017821
References
- ^ Jump up to:a b “Enasidenib”. AdisInsight. Retrieved 31 January 2017.
- Jump up^ https://pubchem.ncbi.nlm.nih.gov/compound/Enasidenib
- Jump up^ https://www.sec.gov/Archives/edgar/data/1439222/000119312516758835/d172494d10q.htm
- Jump up^ http://www.xconomy.com/boston/2016/09/07/celgene-plots-speedy-fda-filing-for-agios-blood-cancer-drug/
-

The U.S. Food and Drug Administration today approved Idhifa (enasidenib) for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) who have a specific genetic mutation. The drug is approved for use with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect specific mutations in the IDH2 gene in patients with AML.
“Idhifa is a targeted therapy that fills an unmet need for patients with relapsed or refractory AML who have an IDH2 mutation,” said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “The use of Idhifa was associated with a complete remission in some patients and a reduction in the need for both red cell and platelet transfusions.”
AML is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of abnormal white blood cells in the bloodstream and bone marrow. The National Cancer Institute at the National Institutes of Health estimates that approximately 21,380 people will be diagnosed with AML this year; approximately 10,590 patients with AML will die of the disease in 2017.
Idhifa is an isocitrate dehydrogenase-2 inhibitor that works by blocking several enzymes that promote cell growth. If the IDH2 mutation is detected in blood or bone marrow samples using the RealTime IDH2 Assay, the patient may be eligible for treatment with Idhifa.
The efficacy of Idhifa was studied in a single-arm trial of 199 patients with relapsed or refractory AML who had IDH2 mutations as detected by the RealTime IDH2 Assay. The trial measured the percentage of patients with no evidence of disease and full recovery of blood counts after treatment (complete remission or CR), as well as patients with no evidence of disease and partial recovery of blood counts after treatment (complete remission with partial hematologic recovery or CRh). With a minimum of six months of treatment, 19 percent of patients experienced CR for a median 8.2 months, and 4 percent of patients experienced CRh for a median 9.6 months. Of the 157 patients who required transfusions of blood or platelets due to AML at the start of the study, 34 percent no longer required transfusions after treatment with Idhifa.
Common side effects of Idhifa include nausea, vomiting, diarrhea, increased levels of bilirubin (substance found in bile) and decreased appetite. Women who are pregnant or breastfeeding should not take Idhifa because it may cause harm to a developing fetus or a newborn baby.
The prescribing information for Idhifa includes a boxed warning that an adverse reaction known as differentiation syndrome can occur and can be fatal if not treated. Sign and symptoms of differentiation syndrome may include fever, difficulty breathing (dyspnea), acute respiratory distress, inflammation in the lungs (radiographic pulmonary infiltrates), fluid around the lungs or heart (pleural or pericardial effusions), rapid weight gain, swelling (peripheral edema) or liver (hepatic), kidney (renal) or multi-organ dysfunction. At first suspicion of symptoms, doctors should treat patients with corticosteroids and monitor patients closely until symptoms go away.
Idhifa was granted Priority Review designation, under which the FDA’s goal is to take action on an application within six months where the agency determines that the drug, if approved, would significantly improve the safety or effectiveness of treating, diagnosing or preventing a serious condition. Idhifa also received Orphan Drugdesignation, which provides incentives to assist and encourage the development of drugs for rare diseases.
The FDA granted the approval of Idhifa to Celgene Corporation. The FDA granted the approval of the RealTime IDH2 Assay to Abbott Laboratories
///////// fda 2017, Idhifa, enasidenib, Энасидениб , إيناسيدينيب ,伊那尼布 , AG 221, fast track designation, orphan drug status , acute myeloid leukemia, CC-90007
CC(C)(CNC1=NC(=NC(=N1)NC2=CC(=NC=C2)C(F)(F)F)C3=NC(=CC=C3)C(F)(F)F)O
 |
|
| Identifiers | |
|---|---|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C19H17F6N7O |
| Molar mass | 473.38 g·mol−1 |
| 3D model (JSmol) | |