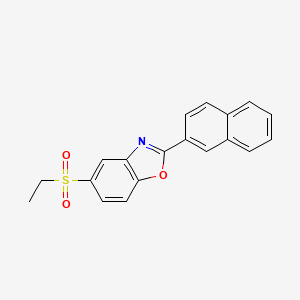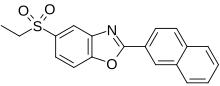
Ezutromid
945531-77-1
Chemical Formula: C19H15NO3S
Molecular Weight: 337.39
945531-77-1, SMT c1100, BMN-195, BMN 195, C 1100
5-(ethylsulfonyl)-2-(naphthalen-2-yl)benzo[d]oxazole
BMN-195; BMN 195; BMN195; SMTC-1100; SMTC1100; SMTC 1100; VOX-C1100; Ezutromid
Ezutromid, also known as BMN-195 and SMTC-1100, is a first orally bioavailable utrophin’s translation modulator. Duchenne muscular dystrophy (DMD) is a lethal, progressive muscle wasting disease caused by a loss of sarcolemmal bound dystrophin, which results in the death of the muscle fibers leading to the gradual depletion of skeletal muscle.
Ezutromid is an orally administered small molecule utrophin modulator currently involved in a Phase 2 clinical trial produced by Summit Therapeutics for the treatment of Duchenne muscular dystrophy (DMD).[1][2] DMD is a fatal x-linked recessive disease affecting approximately 1 in 5000 males and is a designated orphan disease by the FDA and European Medicines Agency.[3] Approximately 1/3 of the children obtain DMD as a result of spontaneous mutation in the dystrophin gene and have no family history of the disease.[3] Dystrophin is a vital component of mature muscle function, and therefore DMD patients have multifarious forms of defunct or deficient dystrophin proteins that all manifest symptomatically as muscle necrosis and eventually organ failure.[3][4] Ezutromid is theorized to maintain utrophin, a protein functionally and structurally similar to dystrophin that precedes and is replaced by dystrophin during development.[3][5] Utrophin and dystrophin are reciprocally expressed, and are found in different locations in a mature muscle cell.[4][6] However, in dystrophin-deficient patients, utrophin was found to be upregulated and is theorized to replace dystrophin in order to maintain muscle fibers.[7] Ezutromid is projected to have the potential to treat all patients suffering with DMD as it maintains the production of utrophin to counteract the lack of dystrophin to retard muscle degeneration.[7][8] Both the FDA and European Medicines Agency has given ezutromid an orphan drug designation.[5][9] The FDA Office of Orphan Products and Development offers an Orphan Drug Designation program (ODD) that allows drugs aimed to treat diseases that affect less than 200,000 people in the U.S. monetary incentives such as a period of market exclusivity, tax incentives, and expedited approval processes.[5][10]
The Phase 2 clinical trial was ended in 2018 and the medication discontinued after it failed to show any benefit in slowing the disease.[11]
Clinical trials
The first Phase 1b trial (NCT02056808) began on November 2013 and involved 12 patients aged 5–11 years old.[12] The patients were divided into three groups given escalating oral doses testing the safety and tolerability after each increase over the course of 10 days.[12]
Another completed Phase 1b trial (NCT02383511) began February 2015 and involved 12 patients aged 5–13 years old.[13] The goal was to determine the safety, tolerability, and pharmacokinetic parameters by measuring plasma concentration and major metabolite levels over 28 days for three sequence groups.[13] Each sequence involved placebo, 1250 mg, and 2500 mg BID (twice a day) doses given for one week each.[4][13]
A PhaseOut DMD, Phase 2, Proof of Concept (NCT02858362) clinical trial is underway that tests the clinical safety and efficacy of an oral suspension of ezutromid.[2] The 48-week open-label trial is enrolling 40 boys, ages 5–10, living in the U.K. or U.S.[2] MRI leg muscle change will be measured as well as ezutromid plasma concentration levels, with a secondary goal of obtaining quantifiable images of utrophin membrane stained biopsies at baseline and either 24 or 48 weeks.[2]
Commercial aspects
As of 2016, ataluren was the only approved drug in the EU to treat a specific subpopulation of patients with nmDMD, or DMD caused by a nonsense mutation.[14] However, nonsense mutations only account for approximately 15% of all patients with DMD.[15] Therefore, Summit Therapeutics projects to file for regulatory approval in the US and EU by 2019 and to reach market in 2020.[8] They expect to profit just over £24,046 in 2020 and £942,656 in 2025, which amounts to ~10% CGR for the first 7 years on the basis of treating all DMD patients in the US, EU, Iceland, Norway, Switzerland and Russia.[8]
Furthermore, Summit Therapeutics has entered an agreement with Sarepta Theraputics as of October 2016 regarding the commercialization of ezutromid.[16] The agreement consists of a collaboration between Sarepta and Summit to share the research and developing costs for the development of novel therapies to treat DMD patients.[16]
PAPER
https://onlinelibrary.wiley.com/doi/10.1002/anie.201906080
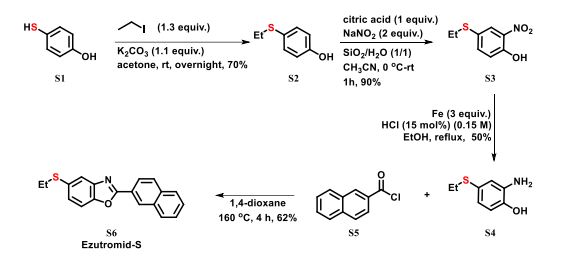
4-(ethylthio)Phenol S2: To a 250 mL round bottle, 4-mercaptophenol S1 (12.6 g, 100 mmol), K2CO3 (15.3 g, 110 mmol), acetone (100 mL) were added, then, iodoethane (15.6 g, 8.0 mL, 130 mmol) was added slowly at 0 oC. The system was stirred at room temperature overnight. After filtration, distillation of solvent, and flash chromatography, S2 (10.780 g) was obtained with 70% yield.
4-(ethylthio)-2-Nitrophenol S3: To a 250 mL round bottle, 4-(ethylthio)Phenol S2 (3.084 g, 20 mmol), 300-400 mesh silica gel (2 g), distilled water (2 g), and CH3CN (60 mL) was added. The system was then cooled by an ice water bath. Subsequently, citric acid (3.842 g, 20 mmol), NaNO2 (2.760 g, 40 mmol) were separately added slowly in portionwise. The system was reacted at room temperature overnight. After filtration and distillation of solvent, EA (50 mL) and water (50 mL) was added, after separation, the aqueous phase was extraction with EA (30 mL) twice. The combined organic phase was dried with MgSO4. Following by filtration and chromatography, S3 (3.590 g) was obtained with 90% yield.
4-(ethylthio)-2-Nitrophenol S4: To a 100 mL round bottle, S3 (2.46 g, 12.3 mmol), reductive iron powder (2.07 g, 36.9 mmol), and EtOH (50 mL) was added. Then, HCl (aq.) (0.15 M) (12 mL, 1.85 mmol) was added slowly. The system was refluxed overnight. After filtration, distillation of solvent, and flash chromatography, S4 (1.040 g) was obtained with 50% yield.
5-(ethylthio)-2-(naphthalen-2-yl)Benzo[d]oxazole S6 (Ezutromid-S): S4 (324 mg1.91 mmol), 2-naphthoyl chloride S5 (545.7 mg, 2.87 mmol), dry 1,4-dioxane (5 mL) was added into a sealing tube. Then, the system was vacuumed and filled with nitrogen for three times. Subsequently, the reaction was run at 160 oC for 10 hours. After distillation of solvent and flash chromatography, S6 (361.7 mg) was obtained with 62% yield. 1H NMR (500 MHz, Chloroform-d) δ 8.74 (s, 1H), 8.28 (dd, J = 8.5, 1.7 Hz, 1H), 7.96 (t, J = 7.5 Hz, 2H), 7.92 – 7.84 (m, 1H), 7.81 (d, J = 1.8 Hz, 1H), 7.57 (pd, J = 6.8, 3.4 Hz, 2H), 7.51 (d, J = 8.3 Hz, 1H), 7.39 (dd, J = 8.4, 1.8 Hz, 1H), 2.99 (q, J = 7.3 Hz, 2H), 1.33 (t, J = 7.3 Hz, 3H).13C NMR (126 MHz, Chloroform-d) δ 163.79, 149.70, 142.92, 134.76, 132.89, 132.38, 128.92, 128.78, 128.20, 127.87, 127.85, 126.91, 124.11, 123.84, 121.38, 110.72, 29.17, 14.40
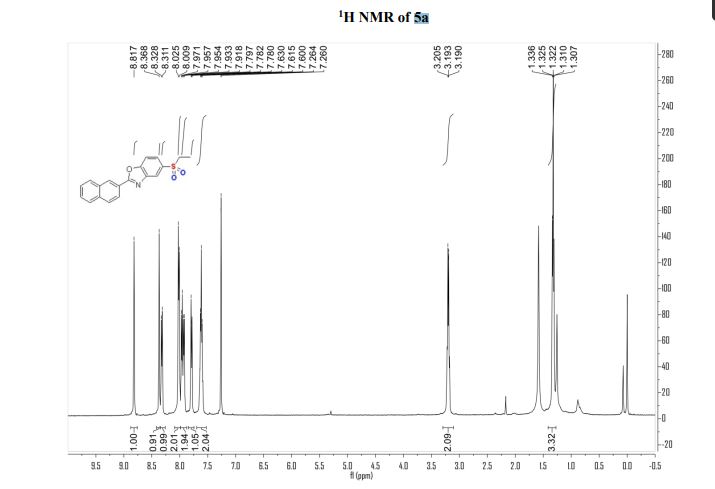
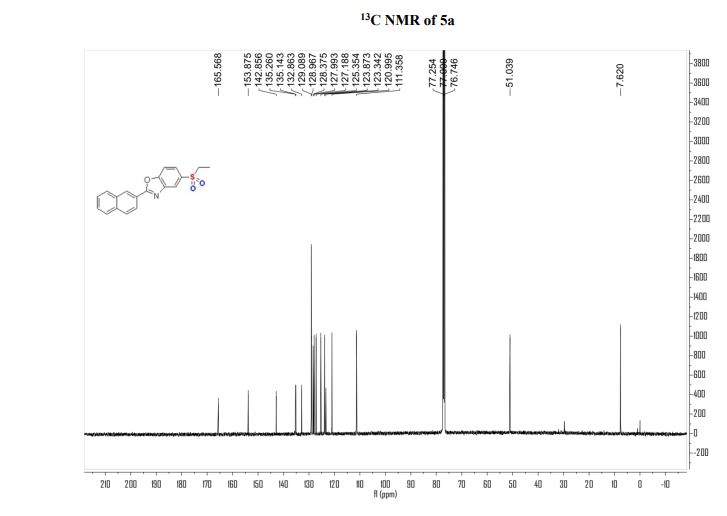
Dibenzoate5-(ethylsulfone)-2-(naphthalen-2- yl)benzo[d]oxazole (Ezotrumid) 5a:
5- (ethylthio)-2-(naphthalen-2-yl)Benzo[d]oxazole (30.5 mg, 0.1 mmol), UO2(OAc)2 . 2H2O (0.8 mg, 0.002 mol), H2O (10 equiv., 36 μL), o-xylene (8.3 equiv., 0.2 mL), CH3CN (1 mL) were stirred under oxygen atmosphere (1 atm, balloon) at room temperature until the total consumption of sulfide and sulfoxide under the irradiation of three 2 w blue LEDs in a paralleled reactor. 5a (27.3 mg, 81%) was obtained through column chromatography (PE/EA = 20/1-5/1) as a white solid, Rf = 0.6 (PE/EA = 2/1);
1H NMR (500 MHz, Chloroform-d) δ 8.82 (s, 1H), 8.37 (s, 1H), 8.32 (d, J = 8.5 Hz, 1H), 8.02 (d, J = 8.0 Hz, 2H), 7.99 – 7.89 (m, 2H), 7.84 – 7.76 (m, 1H), 7.61 (t, J = 7.3 Hz, 2H), 3.28 – 3.08 (m, 2H), 1.32 (dt, J = 7.3, 3.6 Hz, 3H)..
13C NMR (126 MHz, Chloroform-d) δ 165.57, 153.87, 142.86, 135.26, 135.14, 132.86, 129.09, 128.97, 128.37, 127.99, 127.19, 125.35, 123.87, 123.34, 121.00, 111.36, 51.04, 7.62.
IR (KBr) 2933, 1507, 1498, 1258, 1064, 1046, 756, 474 cm-1 .
HRMS (ESI) Calcd for C19H16NO3S 338.0851 (M+H), Found 338.0865.
PATENT
WO 2007091106
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2007091107
PATENT
WO 2009021749
WO 2009019504
WO 2013167737 A
CN 110437170
CN 110483345
CN 110563619
PATENT
WO 2009021748
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2009021748
It has been discovered that the compound of formula I (5-(ethylsulfonyl)-2-(naphthalen-2-yl)benzo[d]oxazole) has excellent properties for the treatment of Duchenne muscular dystrophy (see, e.g., international patent application publication no. WO 2007/091106).
The compound of formula I (R = 5-ethylsulfonyl; R9 = 2-naphthalen-2-yl) may be synthesised according to the following procedure, as disclosed in WO 2007/091106 (page 51):
Experimental
S nthesis of 5- eth lsulfon -2- na hthalen-2- l‘benzo d oxazole
Procedure:
A vessel was equipped with a retreat blade stirrer and downward pumping turbine, a five necked flange lid, seal and clamp, stirrer gland and overhead stirrer, thermometer pocket, Dean- Stark trap, dropping funnel and condenser. The water to the condenser was then switched on.
The sodium hydroxide and 0.80 L of water were then mixed (whilst cooling in an ice bath until all the sodium hydroxide has dissolved – caution exothermic). The resulting solution was then transferred to a scrubber appropriately attached to the vessel.
The 2-amino-4-(ethylsulfonyl)phenol and 2.00 L of xylenes (mixed) were then transferred to the vessel, and the reagents and solvent were stirred at 100 rpm.
Then, the 2-naphtholyl chloride was dissolved in 2.00 L of xylenes (mixed) and transferred into the vessel. The stirring rate was increased to 150 rpm.
The temperature of the solution was gradually increased to 100°C over a period of not less than 30 mins, and then maintained at that level for 10 mins. (Caution: HCl gas is evolved during this process through the gas scrubber). The stirrer speed was then increased to 315 rpm and the temperature gradually increased over a period of 30 minutes until reflux (155°C) at which level it was maintained for 90 mins. (Caution: HCl gas is evolved during this process through the gas scrubber).
The methanesulfonic acid was then added drop-wise over a period of 30 mins and relux was maintained until no further water was being collected in the Dean-Stark apparatus (approx 15 mins).
The heat was then removed and the pipe adapter from the Dean- Stark apparatus disconnected. The resulting solution was allowed to cool to 900C, and then filtered using Whatman 1 filter paper.
The resulting solution was then left at ambient temperature for 18h, after which time the product crystallised, and the product was separated by filtration using Whatman 1 filter paper. The product was then washed with Ix 1.0 L of tert-butyl methyl ether (TBME)
The product was then dried in a vacuum oven at 65°C at a pressure of 1 Ombar until constant weight was achieved (less than 0.5 g difference between consecutive measurements of mass which must be at least 1 h apart).
The product was obtained as a sandy-beige powder in a yield of 80%.
Characterisation:
5-(EthylsuIf onyl)-2-(naphthalen-2-yl)benzo [d] oxazole
LCMS RT= 6.94min, MH+ 338.1;
1H NMR (DMSO): 8.90 (IH, br), 8.34 (IH, d, J 1.4 Hz), 8.30 (IH, dd, J 8.6 1.7 Hz), 8.24-8.05 (4H, m), 7.99 (IH, dd, J 8.5 1.8 Hz), 7.73-7.64 (2H, m), 3.41 (2H, q, J 7.3 Hz), 1.15 (3H, t, J7.3 Hz);
MP = 160-1610C.
Synthesis of polymorphic forms
1. Procedure
100 mg of the compound of formula I was dissolved in the minimum amount of good solvent and then the anti-solvent was added to induce crystallisation. The supernatant liquor was then removed, and the resulting solid was dried under vacuum for 12 his.
PAPER
Journal of medicinal chemistry (2011), 54(9), 3241-50
https://pubs.acs.org/doi/10.1021/jm200135z
Abstract

A series of novel 2-arylbenzoxazoles that upregulate the production of utrophin in murine H2K cells, as assessed using a luciferase reporter linked assay, have been identified. This compound class appears to hold considerable promise as a potential treatment for Duchenne muscular dystrophy. Following the delineation of structure–activity relationships in the series, a number of potent upregulators were identified, and preliminary ADME evaluation is described. These studies have resulted in the identification of 1, a compound that has been progressed to clinical trials.
PAPER
Angewandte Chemie, International Edition (2019), 58(38), 13499-13506
Angewandte Chemie, International Edition (2020), 59(3), 1346-1353.
PAPER
https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.9b01547
Journal of medicinal chemistry (2020), 63(5), 2547-2556.
Abstract

5-(Ethylsulfonyl)-2-(naphthalen-2-yl)benzo[d]oxazole (ezutromid, 1) is a first-in-class utrophin modulator that has been evaluated in a phase 2 clinical study for the treatment of Duchenne muscular dystrophy (DMD). Ezutromid was found to undergo hepatic oxidation of its 2-naphthyl substituent to produce two regioisomeric 1,2-dihydronaphthalene-1,2-diols, DHD1 and DHD3, as the major metabolites after oral administration in humans and rodents. In many patients, plasma levels of the DHD metabolites were found to exceed those of ezutromid. Herein, we describe the structural elucidation of the main metabolites of ezutromid, the regio- and relative stereochemical assignments of DHD1 and DHD3, their de novo chemical synthesis, and their production in systems in vitro. We further elucidate the likely metabolic pathway and CYP isoforms responsible for DHD1 and DHD3 production and characterize their physicochemical, ADME, and pharmacological properties and their preliminary toxicological profiles.
PAPER
https://www.sciencedirect.com/science/article/abs/pii/S004040201931227X
Abstract
Following on from ezutromid, the first-in-class benzoxazole utrophin modulator that progressed to Phase 2 clinical trials for the treatment of Duchenne muscular dystrophy, a new chemotype was designed to optimise its physicochemical and ADME profile. Herein we report the synthesis of SMT022357, a second generation utrophin modulator preclinical candidate, and an asymmetric synthesis of its constituent enantiomers. The pharmacological properties of both enantiomers were evaluated in vitro and in vivo. No significant difference in the activity or efficacy was observed between the two enantiomers; activity was found to be comparable to the racemic mixture.
Graphical abstract


References
- ^ “About Summit Therapeutics – Summit”. Summit. Retrieved 2016-11-14.
- ^ Jump up to:a b c d Clinical trial number NCT02858362 for “PoC Study to Assess Activity and Safety of SMT C1100 (Ezutromid) in Boys With DMD” at ClinicalTrials.gov
- ^ Jump up to:a b c d “Duchenne Muscular Dystrophy – Summit”. Summit. Archived from the original on 2016-11-15. Retrieved 2016-11-14.
- ^ Jump up to:a b c Ricotti V, Spinty S, Roper H, Hughes I, Tejura B, Robinson N, et al. (2016-01-01). “Safety, Tolerability, and Pharmacokinetics of SMT C1100, a 2-Arylbenzoxazole Utrophin Modulator, following Single- and Multiple-Dose Administration to Pediatric Patients with Duchenne Muscular Dystrophy”. PLOS ONE. 11 (4): e0152840. Bibcode:2016PLoSO..1152840R. doi:10.1371/journal.pone.0152840. PMC 4824384. PMID 27055247.
- ^ Jump up to:a b c “Potential DMD Therapy, Ezutromid, Shows Promise in Upgraded Form”. Retrieved 2016-11-14.
- ^ Janghra N, Morgan JE, Sewry CA, Wilson FX, Davies KE, Muntoni F, Tinsley J (2016-03-14). “Correlation of Utrophin Levels with the Dystrophin Protein Complex and Muscle Fibre Regeneration in Duchenne and Becker Muscular Dystrophy Muscle Biopsies”. PLOS ONE. 11 (3): e0150818. Bibcode:2016PLoSO..1150818J. doi:10.1371/journal.pone.0150818. PMC 4790853. PMID 26974331.
- ^ Jump up to:a b “Home – Summit”. Summit. Retrieved 2016-11-14.
- ^ Jump up to:a b c Werther CA (2016). Ezutromid Has the Potential to Treat All Duchenne Patients; Initiating Coverage With a Buy. H.C. Wainwright & Co. pp. 1–29.
- ^ “Search Orphan Drug Designations and Approvals”. www.accessdata.fda.gov. Retrieved 2016-11-14.
- ^ Office of the Commissioner. “Developing Products for Rare Diseases & Conditions”. www.fda.gov. Retrieved 2016-11-14.
- ^ Inacio P (2018-06-29). “Summit Therapeutics Ends Development of Ezutromid Therapy for DMD After Trial Failure”. Muscular Dystrophy News. Retrieved 2019-11-17.
- ^ Jump up to:a b Clinical trial number NCT02056808 for “A Phase 1b Study of SMT C1100 in Subjects With Duchenne Muscular Dystrophy (DMD)” at ClinicalTrials.gov
- ^ Jump up to:a b c Clinical trial number NCT02383511 for “Modified Diet Trial: A Study of SMT C1100 in Paediatric Patients With DMD Who Follow a Balanced Diet ” at ClinicalTrials.gov
- ^ “PTC Therapeutics | ataluren”. PTC Therapeutics. Retrieved 2016-11-15.
- ^ Flanigan KM, Dunn DM, von Niederhausern A, Soltanzadeh P, Howard MT, Sampson JB, et al. (March 2011). “Nonsense mutation-associated Becker muscular dystrophy: interplay between exon definition and splicing regulatory elements within the DMD gene”. Human Mutation. 32 (3): 299–308. doi:10.1002/humu.21426. PMC 3724403. PMID 21972111.
- ^ Jump up to:a b Summit Therapeutics PLC. “Sarepta Therapeutics and Summit Enter Into Exclusive License and Collaboration Agreement for European Rights to Summit’s Utrophin Modulator Pipeline for the Treatment of Duchenne Muscular Dystrophy”. GlobeNewswire News Room. Retrieved 2016-11-15.
/////////Ezutromid, BMN-195, BMN 195, BMN195, SMTC-1100, SMTC1100, SMTC 1100, VOX-C1100, Ezutromid
O=S(C1=CC=C(OC(C2=CC=C3C=CC=CC3=C2)=N4)C4=C1)(CC)=O