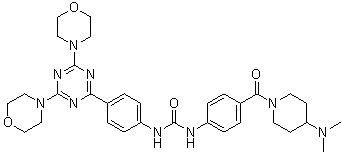
Gedatolisib
Pfizer
PF-05212384; PF-5212384; PKI-587
CAS 1197160-78-3
Chemical Formula: C32H41N9O4
Molecular Weight: 615.72
- Phase III Acute myeloid leukaemia
- Phase II Colorectal cancer; Non-small cell lung cancer
- Phase I Breast cancer; Solid tumours
- Discontinued Endometrial cancer
Most Recent Events
- 22 Nov 2017Pfizer suspends patient enrolment in a phase I/II trial due to drug supply delay in Non-small cell lung cancer (Combination therapy, Inoperable/Unresectable, Metastatic disease, Late-stage disease) in USA (IV) (NCT02920450)
- 04 Nov 2017No recent reports of development identified for phase-I development in Solid-tumours(Combination therapy, Late-stage disease, Second-line therapy or greater) in Canada (IV, Infusion)
- 04 Nov 2017No recent reports of development identified for phase-I development in Solid-tumours(Combination therapy, Late-stage disease, Second-line therapy or greater) in Italy (IV, Infusion)
Gedatolisib, also known as PKI-587 and PF-05212384, is an agent targeting the phosphatidylinositol 3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in the PI3K/mTOR signaling pathway, with potential antineoplastic activity. Upon intravenous administration, PI3K/mTOR kinase inhibitor PKI-587 inhibits both PI3K and mTOR kinases, which may result in apoptosis and growth inhibition of cancer cells overexpressing PI3K/mTOR. Activation of the PI3K/mTOR pathway promotes cell growth, survival, and resistance to chemotherapy and radiotherapy; mTOR, a serine/threonine kinase downstream of PI3K, may also be activated independent of PI3K.
PKI-587 is a PI3K/mTOR inhibitor, currently being developed by Pfizer. The PI3K/Akt signaling pathway is a key pathway in cell proliferation, growth, survival, protein synthesis, and glucose metabolism. It has been recognized recently that inhibiting this pathway might provide a viable therapy for cancer. PKI-587 has shown excellent activity in vitro and in vivo, with antitumor efficacy in both subcutaneous and orthotopic xenograft tumor models when administered intravenously.
PATENT
WO 2009143317
WO 2010096619
WO 2012148540
WO 2014151147
PATENT
US 20170119778
PAPER
Journal of Medicinal Chemistry (2010), 53(6), 2636-2645
http://pubs.acs.org/doi/abs/10.1021/jm901830p
Bis(morpholino-1,3,5-triazine) Derivatives: Potent Adenosine 5′-Triphosphate Competitive Phosphatidylinositol-3-kinase/Mammalian Target of Rapamycin Inhibitors: Discovery of Compound 26 (PKI-587), a Highly Efficacious Dual Inhibitor
Abstract

The PI3K/Akt signaling pathway is a key pathway in cell proliferation, growth, survival, protein synthesis, and glucose metabolism. It has been recognized recently that inhibiting this pathway might provide a viable therapy for cancer. A series of bis(morpholino-1,3,5-triazine) derivatives were prepared and optimized to provide the highly efficacious PI3K/mTOR inhibitor 1-(4-{[4-(dimethylamino)piperidin-1-yl]carbonyl}phenyl)-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea 26 (PKI-587). Compound 26 has shown excellent activity in vitro and in vivo, with antitumor efficacy in both subcutaneous and orthotopic xenograft tumor models when administered intravenously. The structure−activity relationships and the in vitro and in vivo activity of analogues in this series are described.
Preparation of 1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}phenyl)-3-[4-(4,6-dimorpholin-4- yl-1,3,5-triazin-2-yl)phenyl]urea (26)
PAPER
Bioorganic & Medicinal Chemistry Letters (2011), 21(16), 4773-4778.
http://www.sciencedirect.com/science/article/pii/S0960894X11008468
PAPER
New and Practical Synthesis of Gedatolisib
http://pubs.acs.org/doi/10.1021/acs.oprd.7b00298
Abstract
A new, practical, and convergent synthetic route of gedatolisib, an antitumor agent, is developed on a hectogram scale which avoids the Pd coupling method. The key step is adopting 6-(4-nitrophenyl)-1,3,5-triazine-2,4-diamine and 2,2′-dichlorodiethyl ether to prepare the key 4,4′-(6-(4-nitrophenyl)-1,3,5-triazine-2,4-diyl)dimorpholine in 77% yield and 98.8% purity. Gedatolisib is obtained in 48.6% yield over five simple steps and 99.3% purity (HPLC). Purification methods of the intermediates and the final product involved in the route are given.
off-white solid. 1H NMR (400 MHz, DMSO-d6): δ 1.46 (brs, 2H), 1.89 (brs, 2H), 2.29 (s, 6H), 2.94 (brs, 2H), 3.76 (m, 8H), 3.89 (m, 8H), 7.09 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.7 Hz, 2H), 8.28 (s, 1H), 8.31 (d, J = 8.6 Hz, 2H), 8.48 (s, 1H). ESI-MS (m/z) 615.9 (M + H). HPLC conditions: Column: Agilent Eclipse XDB-C18 (250 mm × 4.6 mm × 5 μm); Detection: 254 nm; Flow rate: 0.8 mL/min; Temperature: 30 °C; Injection load: 1 μL; Solvent: MeOH; Concentration: 0.5 mg/mL; Run time: 20 min; Mobile phase A: water; Mobile phase B: MeOH/TEA = 100:0.1; Gradient program: time (min): 20; % of mobile phase A: 10; % of mobile phase B: 90; tR = 2.598 min, purity: 99.34%
- Zhao, X.; Tan, Q.; Zhang, Z.; Zhao, Y. Med. Chem. Res. 2014, 23, 5188– 5196 DOI: 10.1007/s00044-014-1084-z
- Khafizova, G.; Potoski, J. R. PCT Int. Appl. WO 2010096619, 2010.
- Venkatesan, A. M.; Chen, Z.; Dehnhardt, C. M.; Dos Santos, O.; Delos Santos, E. G.; Zask, A.; Verheijen, J. C.; Kaplan, J. A.; Richard, D. J.; Ayral-Kaloustian, S.; Mansour, T. S.; Gopalsamy, A.; Curran, K. J.; Shi, M. PCT Int. Appl. WO 2009143317, 2009.
REFERENCES
1: Gedaly R, Galuppo R, Musgrave Y, Angulo P, Hundley J, Shah M, Daily MF, Chen C, Cohen DA, Spear BT, Evers BM. PKI-587 and sorafenib alone and in combination on inhibition of liver cancer stem cell proliferation. J Surg Res. 2013 Nov;185(1):225-30. doi: 10.1016/j.jss.2013.05.016. Epub 2013 May 25. PubMed PMID: 23769634.
2: Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012 Aug;176(2):542-8. doi: 10.1016/j.jss.2011.10.045. Epub 2011 Nov 21. PubMed PMID: 22261591.
3: Dehnhardt CM, Venkatesan AM, Chen Z, Delos-Santos E, Ayral-Kaloustian S, Brooijmans N, Yu K, Hollander I, Feldberg L, Lucas J, Mallon R. Identification of 2-oxatriazines as highly potent pan-PI3K/mTOR dual inhibitors. Bioorg Med Chem Lett. 2011 Aug 15;21(16):4773-8. doi: 10.1016/j.bmcl.2011.06.063. Epub 2011 Jun 21. PubMed PMID: 21763134.
4: Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, Chen Z, dos Santos O, Ayral-Kaloustian S, Venkatesan A, Hollander I. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res. 2011 May 15;17(10):3193-203. doi: 10.1158/1078-0432.CCR-10-1694. Epub 2011 Feb 15. PubMed PMID: 21325073.
5: Venkatesan AM, Chen Z, dos Santos O, Dehnhardt C, Santos ED, Ayral-Kaloustian S, Mallon R, Hollander I, Feldberg L, Lucas J, Yu K, Chaudhary I, Mansour TS. PKI-179: an orally efficacious dual phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor. Bioorg Med Chem Lett. 2010 Oct 1;20(19):5869-73. doi: 10.1016/j.bmcl.2010.07.104. Epub 2010 Jul 30. PubMed PMID: 20797855.
6: Venkatesan AM, Dehnhardt CM, Delos Santos E, Chen Z, Dos Santos O, Ayral-Kaloustian S, Khafizova G, Brooijmans N, Mallon R, Hollander I, Feldberg L, Lucas J, Yu K, Gibbons J, Abraham RT, Chaudhary I, Mansour TS. Bis(morpholino-1,3,5-triazine) derivatives: potent adenosine 5′-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem. 2010 Mar 25;53(6):2636-45. doi: 10.1021/jm901830p. PubMed PMID: 20166697.
????????????PF 05212384, PF 5212384, PKI-587, PF-05212384; PF-5212384; PKI 587, gedatolisib, antitumor agent, PHASE 3, PFIZER, гедатолисиб , غيداتوليسيب , 吉达利塞 ,
O=C(NC1=CC=C(C2=NC(N3CCOCC3)=NC(N4CCOCC4)=N2)C=C1)NC5=CC=C(C(N6CCC(N(C)C)CC6)=O)C=C5
Journal of Medicinal Chemistry (2017), 60(17), 7524-7538 PQR 309
















