Igermetostat
C32H46N4O4. 550.73
2409538-60-7
2KES6YSH8D
INN 12655
BENZAMIDE, N-((1,2-DIHYDRO-4,6-DIMETHYL-2-OXO-3-PYRIDINYL)METHYL)-3-(ETHYL(TETRAHYDRO-2H-PYRAN-4-YL)AMINO)-2-METHYL-5-((TRANS-3-(1-PIPERIDINYL)CYCLOBUTYL)OXY)-
IGERMETOSTAT
IGERMETOSTAT [INN]
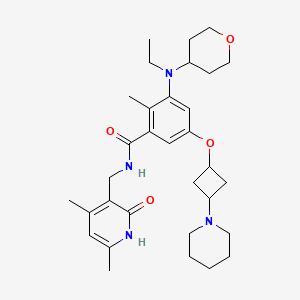
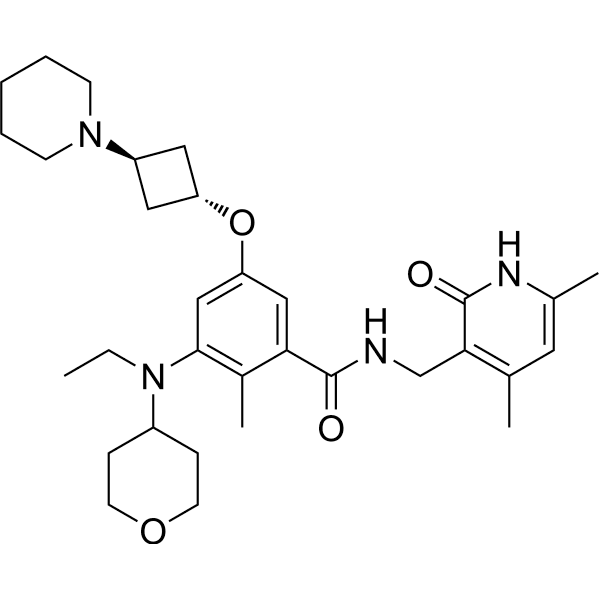
N-((1,2-DIHYDRO-4,6-DIMETHYL-2-OXO-3-PYRIDINYL)METHYL)-3-(ETHYL(TETRAHYDRO-2H-PYRAN-4-YL)AMINO)-2-METHYL-5-((TRANS-3-(1-PIPERIDINYL)CYCLOBUTYL)OXY)BENZAMIDE
N-((4,6-DIMETHYL-2-OXO-1,2-DIHYDROPYRIDIN-3-YL)METHYL)-3-(ETHYL(OXAN-4-YL)AMINO)-2-METHYL-5-(((1R,3R)-3-(PIPERIDIN-1-YL)CYCLOBUTYL)OXY)BENZAMIDE
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020020374&_cid=P22-LR1SQG-34075-1
PATENT
US20230382898
https://patentscope.wipo.int/search/en/detail.jsf?docId=US415363922&_cid=P22-LR1S46-25135-1
The compound has been disclosed in patent WO2020020374A1. It is an enhancer of Zeste homolog 2 (EZH2) inhibitor, and can be used for preventing or treating EZH2-mediated diseases, including brain cancer, thyroid cancer, cardiac sarcoma, lung cancer, oral cancer, stomach cancer and various other cancers.
PATENT
US20210308141
https://patentscope.wipo.int/search/en/detail.jsf?docId=US338556625&_cid=P22-LR1S8P-27079-1
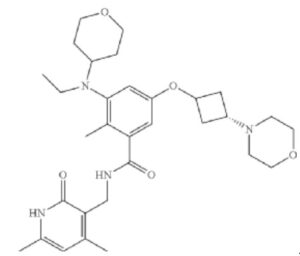
Example 4: Preparation of the Compound N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-2-methyl-5-(trans-3-morpholinylcyclobutoxy)benzamide (4)
[1]. Peng, et al. Polysubstituted benzene compound and preparation method and use thereof.
World Intellectual Property Organization, WO2020020374 A1. 2020-01-30.
//////////Igermetostat
CCN(C1CCOCC1)c2cc(O[C@H]3C[C@@H](C3)N4CCCCC4)cc(C(=O)NCc5c(C)cc(C)[nH]c5=O)c2C