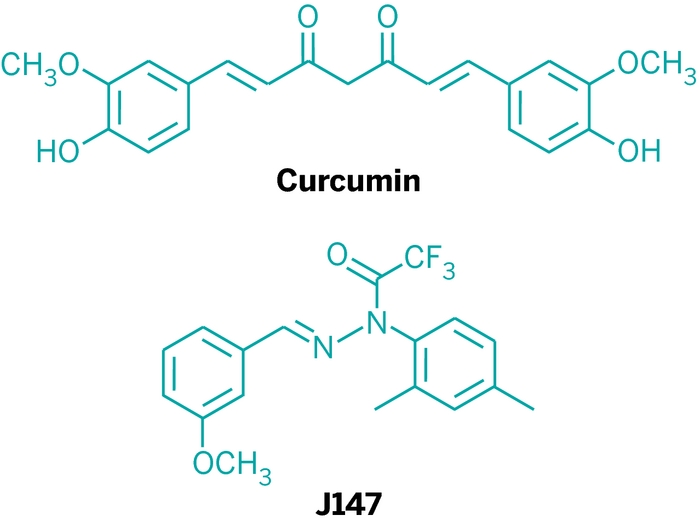
J-147
N-(2,4-Dimethylphenyl)-2,2,2-t
- Molecular FormulaC18H17F3N2O2
- Average mass350.335 Da
2,2,2-trifluoroacetic acid-1-(2,4-dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide
Acetic acid, 2,2,2-
FDA UNII Z41H3C5BT9
Abrexa Pharmaceuticals, Dementia, Alzheimer’s type, PHASE1
Blanchette Rockefeller Neurosci Inst (Originator)
Salk Institute for Biological Studies (Originator)
Abrexa Pharmaceuticals is developing the oral curcumin derivative J-147 for the treatment of Alzheimer’s disease. A phase I clinical trial is under way in healthy young and older adults.
The Salk Institute for Biological Studies and Abrexa Pharmaceuticals are developing J-147, a curcumin derivative CNB-001 , and a 5-lipoxygenase inhibitor, for the oral treatment of Alzheimer’s disease (AD), aging and acute ischemic stroke; in January 2019, a phase I trial for AD was initiated.
J147 is an experimental drug with reported effects against both Alzheimer’s disease and ageing in mouse models of accelerated aging.[1][2][3][4]
The approach that lead to development of the J147 drug was to screen candidate molecules for anti-aging effects, instead of targeting the amyloid plaques. It is contrary to most other approaches to developing drugs against Alzheimer’s disease that target the plaque deposits in the brain.[5]
The J147 drug is also reported to address other biological aging factors, such as preventing the leakage of blood from microvessels in mice brains.[5] The development of J147 follows the chemical pharmacological way, contrary to biological ways that exploit e.g. use of bacteriophages.[6][7]
Enhanced neurogenic activity over J147 in human neural precursor cells has its derivative called CAD-31. CAD-31 is enhancing the use of free fatty acids for energy production by shifting of the metabolic profile of fatty acids toward the production of ketone bodies, a potent source of energy in the brain when glucose levels are low.[8]
The target molecule is a protein called ATP synthase, which is found in the mitochondria.[9]
PAPER
Organic & Biomolecular Chemistry (2015), 13(37), 9564-9569
https://pubs.rsc.org/en/content/articlelanding/2015/OB/C5OB01463H#!divAbstract
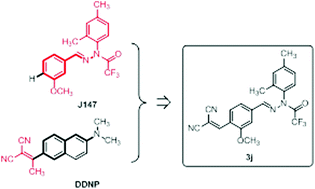
A series of novel J147 derivatives were synthesized, and their inhibitory activities against β-amyloid (Aβ) aggregation and toxicity were evaluated by using the oligomer-specific antibody assay, the thioflavin-T fluorescence assay, and a cell viability assay in the transformed SH-SY5Y cell culture. Among the synthesized J147 derivatives, 3j with a 2,2-dicyanovinyl substituent showed the most potent inhibitory activity against Aβ42oligomerization (IC50 = 17.3 μM) and Aβ42 fibrillization (IC50 = 10.5 μM), and disassembled the preformed Aβ42 fibrils with an EC50 of 10.2 μM. Finally, we confirmed that 3j is also effective at preventing neurotoxicity induced by Aβ42-oligomers as well as Aβ42-fibrils.
PAPER
https://www.sciencedirect.com/science/article/pii/S0960894X12014746

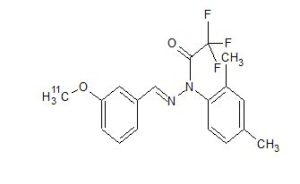
Figure 1. Chemical structures of previously developed [11C]PIB, [18F]Amyvid and [18F]-T808, and newly developed [11C]J147.

Scheme 1. Synthesis of the reference standard J147 (2).
PRODUCT PATENT
WO2009052116
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2009052116&tab=PCTDESCRIPTION
PATENT
WO-2019164997
A process for preparing crystalline Form II of 2,2,2-trifluoroacetic acid-1-(2,4-dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide (J-147; 98% of purity) comprising the steps of providing a slurry containing saturated amorphous or crystalline Form I of J-147 and mixing the slurry to obtain the crystalline Form II of J147. Also claimed are processes for preparing the crystalline Form I of 2,2,2-trifluoroacetic acid-1-(2,4-dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide. Further claimed are isolation of the crystalline Form II and I of 2,2,2-trifluoroacetic acid-1-(2,4-dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide. The compound is disclosed to be a neurotrophic agent and known to be a Trkb receptor agonist, useful for treating neurodegenerative disease, such as aging and motor neurone disease.
The present disclosure relates to polymorph forms of a pharmaceutical active agent. In particular, the present disclosure relates to polymorph forms of neuroprotective agent 2,2,2-trifluoroacetic acid l-(2,4-Dimethylphenyl)-2-[(3-methoxyphenyl)methylene] hydrazide (J147).
[0002] 2,2,2 -trifluoroacetic acid l-(2,4-Dimethylphenyl)-2-[(3-methoxyphenyl)methylene] hydrazide (J147) is a potent orally active neurotrophic agent discovered during screening for efficacy in cellular models of age-associated pathologies and has a structure given by Formula I:
[0003] J147 is broadly neuroprotective, and exhibited activity in assays indicating distinct neurotoxicity pathways related to aging and neurodegenerative diseases, with EC50 between 10 and 200 nM. It has been indicated to improve memory in normal rodents, and prevent the loss of synaptic proteins and cognitive decline in a transgenic AD mouse model.
Furthermore, it has displayed neuroprotective, neuroanti-inflammatory, and LTP-enhancing activity.
[0004] The neurotrophic and nootropic effects have been associated with increases in BDNF levels and BDNF responsive proteins. Interestingly, despite this mechanism of action, Jl47’s neuroprotective effects have been observed to be independent of TrkB receptor activation.
J147 has been indicated to reduce soluble Ab40 and Ab42 levels, and it is currently being researched for potential applications in treating ALS.
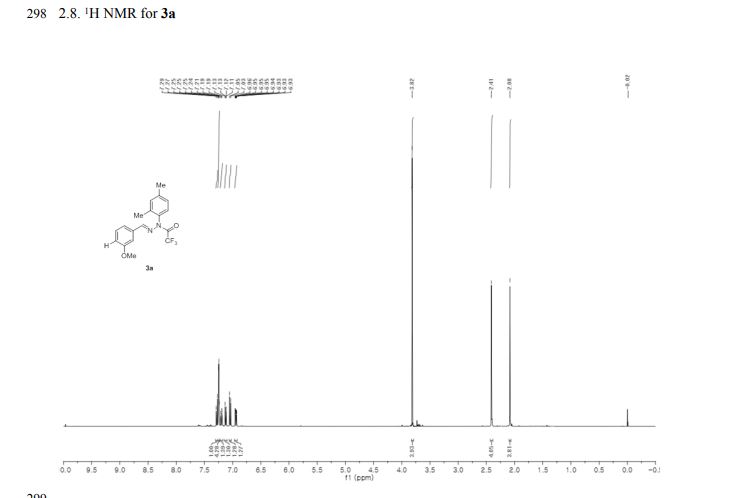
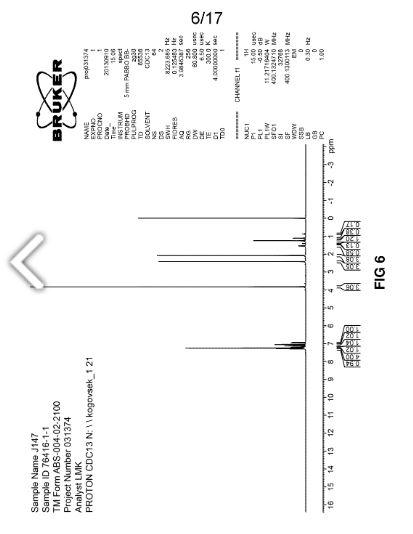
The Fourier transform infrared (FTIR) spectrum is shown in Figure 4. Based on visual inspection the spectrum is consistent with structure. The Raman spectrum is in agreement with the FTIR spectrum and is shown in Figure 5. The proton NMR data is consistent with the structure of J147 and is shown in Figure 6. The proton NMR data is also shown in tabulated form in Table B below.
Table B
EXAMPLE OF PREPARATION OF FORM II OF J 147
Batch Process: About 100 kg of crude J147 from its synthetic preparation was evaporated twice from about 80 kg of ethanol. The crude product was taken up in about 48 kg of ethanol and the batch temperature was adjusted to 28 °C. About 37 kg of water was added gradually to the batch. The batch was held at about 30 °C for about 1.7 hours. A sample of the batch was pulled from the reactor and solids precipitated by addition of 45 mL of water. The solids obtained were added back to the batch as seed crystals and the mixture stirred for 40 minutes at 30 °C. An additional about 34 kg of water was added. The batch was held at about 18 °C for about 58 hours and then cooled to about 10 °C for another about 5.5 hours. Analysis of the resultant solids indicated the presence of Form I. Form I was converted to Form II by heating the slurry to about 45 °C for about 16 hours and then cooling back to about 10 °C and holding the batch at this temperature for about 3 hours about 17.7 kg of solid Form II of J147 were recovered by filtration after washing and drying.
CLIP
https://cen.acs.org/articles/90/i31/Tumeric-Derived-Compound-Curcumin-Treat.html
Turmeric-Derived Compound Curcumin May Treat Alzheimer’s

More than 5 million people in the U.S. currently live with Alzheimer’s disease. And according to the Alzheimer’s Association, the situation is only going to get worse.
By 2050, the nonprofit estimates, up to 16 million Americans will have the memory-robbing disease. It will cost the U.S. $1.1 trillion annually to care for them unless a successful therapy is found.
Pharmaceutical companies have invested heavily in developing Alzheimer’s drugs, many of which target amyloid-β, a peptide that misfolds and clumps in the brains of patients. But so far, no amyloid-β-targeted medications have been successful. Expectation for the most advanced drugs—bapineuzumab from Pfizer and Johnson & Johnson and solanezumab from Eli Lilly & Co.—are low on the basis of lackluster data from midstage clinical trials. That sentiment was reinforced last week when bapineuzumab was reported to have failed the first of four Phase III studies.
Even if these late-stage hopefuls do somehow work, they won’t come cheap, says Gregory M. Cole, a neuroscientist at the University of California, Los Angeles. These drugs “would cost patients tens of thousands of dollars per year,” he estimates. That hefty price tag stems from bapineuzumab and solanezumab being costly-to-manufacture monoclonal antibodies against amyloid-β.
“There’s a great need for inexpensive Alzheimer’s treatments,” as well as a backup plan if pharma fails, says Larry W. Baum, a professor in the School of Pharmacy at the Chinese University of Hong Kong. As a result, he says, a great many researchers have turned their attention to less pricy alternatives, such as compounds from plants and other natural sources.
Curcumin, a spice compound derived from the rootstalk of the turmeric plant (Curcuma longa), has stood out among some of the more promising naturally derived candidates.
When administered to mice that develop Alzheimer’s symptoms, curcumin decreases inflammation and reactive oxygen species in the rodents’ brains, researchers have found. The compound also inhibits the aggregation of troublesome amyloid-β strands among the animals’ nerve cells. But the development of curcumin as an Alzheimer’s drug has been stymied, scientists say, both by its low uptake in the body and a lack of funds for effective clinical trials—obstacles researchers are now trying to overcome.
In addition to contributing to curry dishes’ yellow color and pungent flavor, curcumin has been a medicine in India for thousands of years. Doctors practicing traditional Hindu medicine admire turmeric’s active ingredient for its anti-inflammatory properties and have used it to treat patients for ailments including digestive disorders and joint pain.
Only in the 1970s did Western researchers catch up with Eastern practices and confirm curcumin’s anti-inflammatory properties in the laboratory. Scientists also eventually determined that the polyphenolic compound is an antioxidant and has chemotherapeutic activity.

Bharat B. Aggarwal, a professor at the University of Texas M. D. Anderson Cancer Center, says curcumin is an example of a pleiotropic agent: It has a number of different effects and interacts with many targets and biochemical pathways in the body. He and his group have discovered that one important molecule targeted and subsequently suppressed by curcumin is NF-κB, a transcription factor that switches on the body’s inflammatory response when activated (J. Biol. Chem.,DOI: 10.1074/jbc.270.42.24995).
Aside from NF-κB, curcumin seems to interact with several other molecules in the inflammatory pathway, a biological activity that Aggarwal thinks is advantageous. “All chronic diseases are caused by dysregulation of multiple targets,” he says. “Chemists don’t yet know how to design a drug that hits multiple targets.” With curcumin, “Mother Nature has already provided a compound that does so.”
Curcumin’s pleiotropy also brought it to the attention of UCLA’s Cole during the early 1990s while he was searching for possible Alzheimer’s therapeutics. “That was before we knew about amyloid-β” and its full role in Alzheimer’s, he says. “We were working on the disease from an oxidative damage and inflammation point of view—two processes implicated in aging.”
When Cole and his wife, Sally A. Frautschy, also at UCLA, searched the literature for compounds that could tackle both of these age-related processes, curcumin jumped out at them. It also didn’t hurt that the incidence of Alzheimer’s in India, where large amounts of curcumin are consumed regularly, is lower than in other parts of the developing world (Lancet Neurol., DOI: 10.1016/s1474-4422(08)70169-8).
In 2001, Cole, Frautschy, and colleagues published the first papers that demonstrated curcumin’s potential to treat neurodegenerative disease (Neurobiol. Aging, DOI: 10.1016/s0197-4580(01)00300-1; J. Neurosci.2001, 8370). The researchers studied the effects of curcumin on rats that had amyloid-β injected into their brains, as well as mice engineered to develop amyloid brain plaques. In both cases, curcumin suppressed oxidative tissue damage and reduced amyloid-β deposits.
Those results, Cole says, “turned us into curcumin-ologists.”
Although the UCLA team observed that curcumin decreased amyloid plaques in animal models, at the time, the researchers weren’t sure of the molecular mechanism involved.
Soon after the team’s first results were published, Cole recalls, a colleague brought to his attention the structural similarity between curcumin and the dyes used to stain amyloid plaques in diseased brain tissue. When Cole and Frautschy tested the spice compound, they saw that it, too, could stick to aggregated amyloid-β. “We thought, ‘Wow, not only is curcumin an antioxidant and an anti-inflammatory, but it also might be an anti-amyloid drug,’ ” he says.
In 2004, a group in Japan demonstrated that submicromolar concentrations of curcumin in solution could inhibit aggregation of amyloid-β and break up preformed fibrils of the stuff (J. Neurosci. Res., DOI: 10.1002/jnr.20025). Shortly after that, the UCLA team demonstrated the same (J. Biol. Chem., DOI: 10.1074/jbc.m404751200).
As an Alzheimer’s drug, however, it’s unclear how important it is that the spice compound inhibits amyloid-β aggregation, Cole says. “When you have something that’s so pleiotropic,” he adds, “it’s hard to know” which of its modes of action is most effective.
Having multiple targets may be what helps curcumin have such beneficial, neuroprotective effects, says David R. Schubert, a neurobiologist at the Salk Institute for Biological Studies, in La Jolla, Calif. But its pleiotropy can also be a detriment, he contends.
The pharmaceutical world, Schubert says, focuses on designing drugs aimed at hitting single-target molecules with high affinity. “But we don’t really know what ‘the’ target for curcumin is,” he says, “and we get knocked for it on grant requests.”
Another problem with curcumin is poor bioavailability. When ingested, UCLA’s Cole says, the compound gets converted into other molecular forms, such as curcumin glucuronide or curcumin sulfate. It also gets hydrolyzed at the alkaline and neutral pHs present in many areas of the body. Not much of the curcumin gets into the bloodstream, let alone past the blood-brain barrier, in its pure, active form, he adds.
Unfortunately, neither Cole nor Baum at the Chinese University of Hong Kong realized the poor bioavailability until they had each launched a clinical trial of curcumin. So the studies showed no significant difference between Alzheimer’s patients taking the spice compound and those taking a placebo (J. Clin. Psychopharmacol., DOI: 10.1097/jcp.0b013e318160862c).
“But we did show curcumin was safe for patients,” Baum says, finding a silver lining to the blunder. “We didn’t see any adverse effects even at high doses.”
Some researchers, such as Salk’s Schubert, are tackling curcumin’s low bioavailability by modifying the compound to improve its properties. Schubert and his group have come up with a molecule, called J147, that’s a hybrid of curcumin and cyclohexyl-bisphenol A. Like Cole and coworkers, they also came upon the compound not by initially screening for the ability to interact with amyloid-β, but by screening for the ability to alleviate age-related symptoms.
The researchers hit upon J147 by exposing cultured Alzheimer’s nerve cells to a library of compounds and then measuring changes to levels of biomarkers for oxidative stress, inflammation, and nerve growth. J147 performed well in all categories. And when given to mice engineered to accumulate amyloid-β clumps in their brains, the hybrid molecule prevented memory loss and reduced formation of amyloid plaques over time (PLoS One, DOI: 10.1371/journal.pone.0027865).
Other researchers have tackled curcumin’s poor bioavailability by reformulating it. Both Baum and Cole have encapsulated curcumin in nanospheres coated with either polymers or lipids to protect the compound from modification after ingestion. Cole tells C&EN that by packaging the curcumin in this way, he and his group have gotten micromolar quantities of it into the bloodstream of humans. The researchers are now preparing for a small clinical trial to test the formulation on patients with mild cognitive impairment, who are at an increased risk of developing Alzheimer’s.
An early-intervention human study such as this one comes with its own set of challenges, Cole says. People with mild cognitive impairment “have good days and bad days,” he says. A large trial over a long period would be the best way to get any meaningful data, he adds.
Such a trial can cost up to $100 million, a budget big pharma might be able to scrape together but that is far out of reach for academics funded by grants, Cole says. “If you’re down at the level of what an individual investigator can do, you’re running a small trial,” he says, “and even if the result is positive, it might be inconclusive” because of its small size or short duration. That’s one of the reasons the curcumin work is slow-going, Cole contends.
The lack of hard clinical evidence isn’t stopping people from trying curcumin anyway. Various companies are selling the spice compound as a dietary supplement, both in its powdered form and in nanoformulations such as the ones Cole and Baum are working with. Indiana-based Verdure Sciences, for instance, licensed a curcumin nanoformulation from UCLA and sells it under the name Longvida (about $1.00 to $2.00 per capsule, depending on the distributor).
“There’s no proof that it works,” Cole says. “If you want to take it, you’re experimenting on yourself.” And he cautions that correct dosing for this more bioavailable form of curcumin hasn’t yet been established, so there could be safety concerns.
But on the basis of positive e-mails he’s received from caregivers and Alzheimer’s patients who are desperate for options and trying supplements, “I have some hope,” Cole says. “Maybe there’s something to curcumin after all.”
CLIP

Raw J 147 powder basic Characters
| Name: | J 147 powder |
| CAS: | 1146963-51-0 |
| Molecular Formula: | C18H17F3N2O2 |
| Molecular Weight: | 350.3349896 |
| Melt Point: | 177-178°C |
| Storage Temp: | 4°C |
| Color: | White or off white powder |
Raw J 147 powder in enhance brain function and an extra boost cycle
Names
J 147 powder
J 147 (1146963-51-0) Usage dosage
Using a drug discovery scheme for Alzheimer’s disease (AD) that is based upon multiple pathologies of old age, we identified a potent compound with efficacy in rodent memory and AD animal models. Since this compound, J-147 powder, is a phenyl hydrazide, there was concern that it can be metabolized to aromatic amines/hydrazines that are potentially carcinogenic. To explore this possibility, we examined the metabolites of J 147 powder in human and mouse microsomes and mouse plasma. It is shown that J-147(1146963-51-0) powder is not metabolized to aromatic amines or hydrazines, that the scaffold is exceptionally stable, and that the oxidative metabolites are also neuroprotective. It is concluded that the major metabolites of J 147(1146963-51-0) powder may contribute to its biological activity in animals.
J 147 , derived from the curry spice component curcumin, has low toxicity and actually reverses damage in neurons associated with Alzheimer’s.
J 147 (1146963-51-0) was the mitochondrial protein known as ATP synthase, specifically ATP5A, a subunit of that protein. ATP synthase is involved in the mitochondrial generation of ATP, which cells use for energy.
The researchers demonstrated that by reducing the activity of ATP synthase, they were able to protect neuronal cells from a number of toxicities associated with the aging of the brain. One reason for this neuroprotective effect is thought to be the role of excitotoxicity in neuronal cell damage.
Excitotoxicity is the pathological process by which neurons are damaged and killed by the overactivation of receptors for the excitatory neurotransmitter glutamate. Think of it being a bit like a light switch being turned on and off so rapidly that it ends up causing the light bulb to blow.
Recently, the role of ATP synthase inhibition for neuroprotection against excitotoxic damage was demonstrated in a mouse study[4]. The second study showed that mouse models expressing the human form of mutant ATPase inhibitory factor 1 (hIF1), which causes a sustained inhibition of ATP synthase, were more resilient to neuronal death after excitotoxic damage. This data is consistent with this new J 147 powder study, in which an increase in IF1 in the mice reduced the activity of ATP synthase (specifically ATP5A) and was neuroprotective.
Warning on Raw J 147 powder
Data presented here demonstrate that J-147 powder has the ability to rescue cognitive deficits when administered at a late stage in the disease. The ability of J-147 powder to improve memory in aged AD mice is correlated with its induction of the neurotrophic factors NGF (nerve growth factor) and BDNF (brain derived neurotrophic factor) as well as several BDNF-responsive proteins which are important for learning and memory. The comparison between J-147(1146963-51-0) powder and donepezil in the scopolamine model showed that while both compounds were comparable at rescuing short term memory, J-147 powder was superior at rescuing spatial memory and a combination of the two worked best for contextual and cued memory.
Further instructions
Alzheimer’s disease is a progressive brain disorder, recently ranked as the third leading cause of death in the United States and affecting more than five million Americans. It is also the most common cause of dementia in older adults, according to the National Institutes of Health. While most drugs developed in the past 20 years target the amyloid plaque deposits in the brain (which are a hallmark of the disease), few have proven effective in the clinic.
“While most drugs developed in the past 20 years target the amyloid plaque deposits in the brain (which are a hallmark of the disease), none have proven effective in the clinic,” says Schubert, senior author of the study.
Several years ago, Schubert and his colleagues began to approach the treatment of the disease from a new angle. Rather than target amyloid, the lab decided to zero in on the major risk factor for the disease–old age. Using cell-based screens against old age-associated brain toxicities, they synthesized J 147(1146963-51-0) powder.
Previously, the team found that J-147 powder could prevent and even reverse memory loss and Alzheimer’s pathology in mice that have a version of the inherited form of Alzheimer’s, the most commonly used mouse model. However, this form of the disease comprises only about 1 percent of Alzheimer’s cases. For everyone else, old age is the primary risk factor, says Schubert. The team wanted to explore the effects of the drug candidate on a breed of mice that age rapidly and experience a version of dementia that more closely resembles the age-related human disorder.

References
- ^ “Experimental drug targeting Alzheimer’s disease shows anti-aging effects” (Press release). Salk Institute. 12 November 2015. Retrieved November 13, 2015.
- ^ Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, Schubert D (14 December 2011). “A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease”. PLoS One. 6 (12): e27865. doi:10.1371/journal.pone.0027865. PMC 3237323. PMID 22194796.
- ^ Currais A, Goldberg J, Farrokhi C, Chang M, Prior M, Dargusch R, Daugherty D, Armando A, Quehenberger O, Maher P, Schubert D (11 November 2015). “A comprehensive multiomics approach toward understanding the relationship between aging and dementia” (PDF). Aging. 7 (11): 937–55. doi:10.18632/aging.100838. PMC 4694064. PMID 26564964.
- ^ Prior M, Dargusch R, Ehren JL, Chiruta C, Schubert D (May 2013). “The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer’s disease mice”. Alzheimer’s Research & Therapy. 5 (3): 25. doi:10.1186/alzrt179. PMC 3706879. PMID 23673233.
- ^ Jump up to:a b Brian L. Wang (13 November 2015). “Experimental drug targeting Alzheimer’s disease shows anti-aging effects in animal tests”. nextbigfuture.com. Retrieved November 16, 2015.
- ^ Krishnan R, Tsubery H, Proschitsky MY, Asp E, Lulu M, Gilead S, Gartner M, Waltho JP, Davis PJ, Hounslow AM, Kirschner DA, Inouye H, Myszka DG, Wright J, Solomon B, Fisher RA (2014). “A bacteriophage capsid protein provides a general amyloid interaction motif (GAIM) that binds and remodels misfolded protein assemblies”. Journal of Molecular Biology. 426: 2500–19. doi:10.1016/j.jmb.2014.04.015. PMID 24768993.
- ^ Solomon B (October 2008). “Filamentous bacteriophage as a novel therapeutic tool for Alzheimer’s disease treatment”. Journal of Alzheimer’s Disease. 15 (2): 193–8. PMID 18953108.
- ^ Daugherty, D., Goldberg, J., Fischer, W., Dargusch, R., Maher, P., & Schubert, D. (2017). A novel Alzheimer’s disease drug candidate targeting inflammation and fatty acid metabolism. Alzheimer’s research & therapy, 9(1), 50. https://doi.org/10.1186/s13195-017-0277-3
- ^ “Researchers identify the molecular target of J147, which is nearing clinical trials to treat Alzheimer’s disease”. Retrieved 2018-01-30.
 |
|
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C18H17F3N2O2 |
| Molar mass | 350.341 g·mol−1 |
| 3D model (JSmol) | |
////////////J-147, J 147, J147, Alzheimer’s disease, neurotrophic agent, The Salk Institute for Biological Studies, Abrexa Pharmaceuticals, PHASE 1, CURCUMIN
CAS 1417911-00-2
- Acetic acid, 2,2,2-trifluoro-, 1-(2,4-dimethylphenyl)-2-[[3-(methoxy-11C)phenyl]methylene]hydrazide