
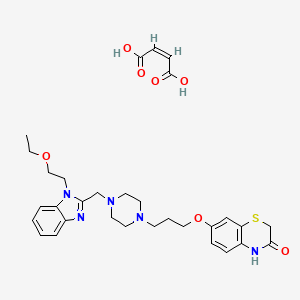
K-8986
(Z)-but-2-enedioic acid;7-[3-[4-[[1-(2-ethoxyethyl)benzimidazol-2-yl]methyl]piperazin-1-yl]propoxy]-4H-1,4-benzothiazin-3-one
cas 1335112-55-4 mono maleate
cas 1335112-57-6 di maleate
cas 219741-69-2 free form
C27 H35 N5 O3 S . C4 H4 O4
2H-1,4-Benzothiazin-3(4H)-one, 7-[3-[4-[[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]methyl]-1-piperazinyl]propoxy]-, (2Z)-2-butenedioate (1:1)
7-[3-[4-[[1-(2-Ethoxyethyl)benzimidazol-2-yl]methyl]-1-piperazinyl]propoxy]-3,4-dihydro-2H-1,4-benzothiazin-3-one monomaleate
KOWA CO., LTD.
| 福田 友昭 FUKUDA, Tomoaki; JP |
| 纐纈 章泰 KOKETSU, Akiyasu; JP |
| 金児 佳生 KANEKO, Yoshio; JP |
| 芦川 由香 ASHIKAWA, Yuka; JP |

Mono maleate
1H NMR (396 MHz, DMSO-d6) δ 1.03 (t, J = 7.0 Hz, 3H), 2.04–2.08 (m, 2H), 3.10 (br, 8H), 3.18 (br, 2H), 3.38 (t, J = 7.0 Hz, 2H), 3.42 (s, 2H), 3.71 (t, J = 7.9 Hz, 2H), 3.95 (s, 2H), 4.01 (t, J = 5.9 Hz, 2H), 4.51 (t, J = 5.2 Hz, 2H), 6.06 (s, 2H), 6.79 (dd, J = 9.1, 2.7 Hz, 1H), 6.90–6.92 (m, 2H), 7.17–7.26 (m, 2H), 7.58–7.61 (m, 2H), 10.43 (s, 1H);
13C NMR (100 MHz, DMSO-d6) δ 15.0, 23.8, 29.0, 43.5, 49.7 (×2), 51.3 (×2), 53.2, 53.4, 65.3, 65.7, 68.7, 110.7, 112.8, 113.9, 118.2, 118.8, 120.3, 121.6, 122.2, 131.3, 135.6, 135.8 (×2), 141.8, 150.6, 153.7, 164.7, 167.3 (×2);
HRMS (FD) calcd for C27H36N5O3S [(MH – maleic acid)+] 510.2539, found 510.2558.
Allergic conjunctivitis, which can be classified into seasonal allergic conjunctivitis and perennial allergic conjunctivitis, is a type I hypersensitivity to allergens. Symptoms such as itching, redness, eyelid swelling, and chemosis are common among afflicted patients and are caused by the release of chemical mediators such as histamine from activated mast cells through cross-linking of antigen-specific immunoglobulin E. The binding of histamine to its receptors plays a central role in the induction of allergic symptoms. K-8986 (1), a histamine H1-receptor antagonist, was developed as a potential therapeutic for treatment of allergic conjunctivitis
SYN
Clip
Development of a Synthetic Process for K-8986, an H1-Receptor Antagonist
Tomoaki Fukuda*†‡  , Takeaki Hara†, Shinji Ina†, Tetsuhiro Nemoto‡
, Takeaki Hara†, Shinji Ina†, Tetsuhiro Nemoto‡  , and Takeshi Oshima*†
, and Takeshi Oshima*†
† Tokyo New Drug Research Laboratories, Pharmaceutical Division, Kowa Company, Ltd., 2-17-43, Noguchicho, Higashimurayama, Tokyo 189-0022, Japan
‡ Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675, Japan
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00380
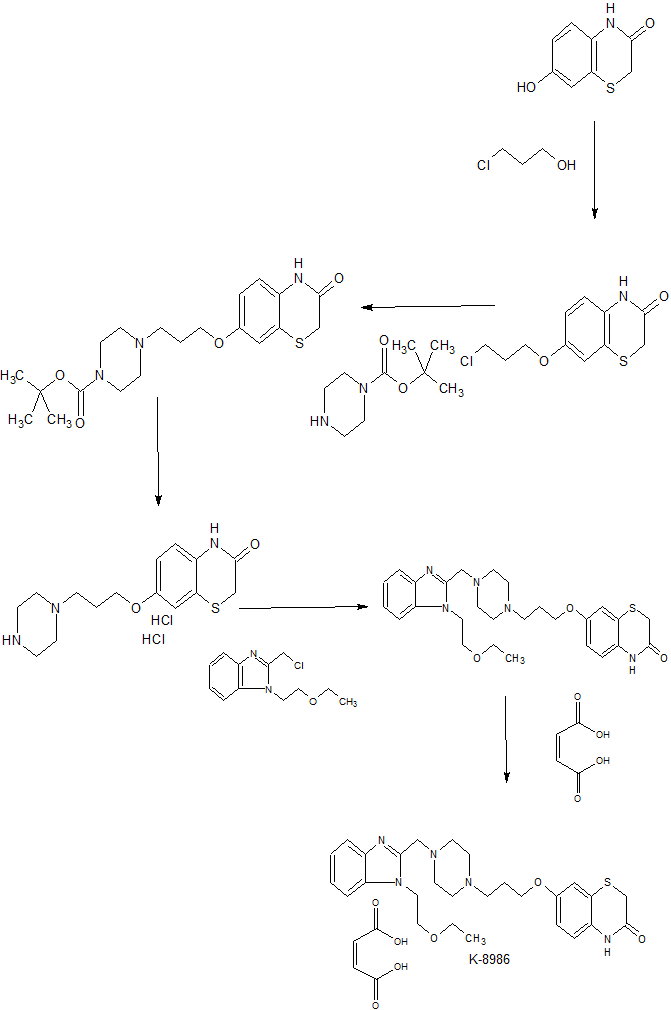
This article describes the development of a robust and scalable synthetic process for K-8986 (1). To solve the problems in terms of the physicochemical properties of 6 (a free base unit of 1), we have screened the suitable salt forms of the target. The monomaleate salt was the most suitable form for the API. To overcome challenges regarding the unremovable impurity Imp B caused by the carryover of piperazine in the medicinal chemistry route, we designed and developed a novel synthetic route. This route furnished more opportunities to purify the synthetic intermediates after introduction of the piperazine unit. Both impurities and co-products in each step of the revised synthesis could be easily removed via filtration, leveraging the low solubility of benzothiazine derivatives. The newly established process was applied to the synthesis of 1 (the monomaleate salt of 6) on a practical scale, achieving high purity and reproducibility.
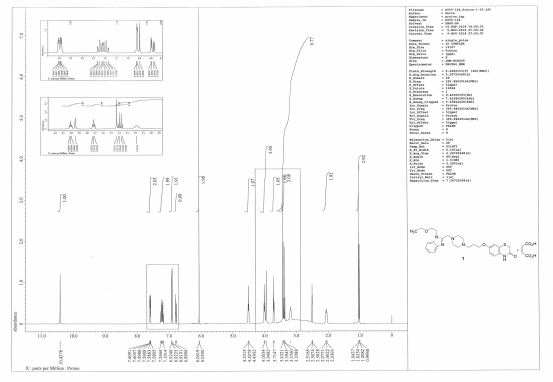
1H NMR (396 MHz, DMSO-d6) δ 1.03 (t, J = 7.0 Hz, 3H), 2.04–2.08 (m, 2H), 3.10 (br, 8H), 3.18 (br, 2H), 3.38 (t, J = 7.0 Hz, 2H), 3.42 (s, 2H), 3.71 (t, J = 7.9 Hz, 2H), 3.95 (s, 2H), 4.01 (t, J = 5.9 Hz, 2H), 4.51 (t, J = 5.2 Hz, 2H), 6.06 (s, 2H), 6.79 (dd, J = 9.1, 2.7 Hz, 1H), 6.90–6.92 (m, 2H), 7.17–7.26 (m, 2H), 7.58–7.61 (m, 2H), 10.43 (s, 1H);
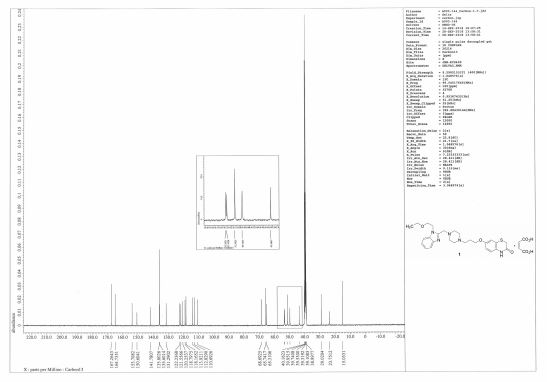
13C NMR (100 MHz, DMSO-d6) δ 15.0, 23.8, 29.0, 43.5, 49.7 (×2), 51.3 (×2), 53.2, 53.4, 65.3, 65.7, 68.7, 110.7, 112.8, 113.9, 118.2, 118.8, 120.3, 121.6, 122.2, 131.3, 135.6, 135.8 (×2), 141.8, 150.6, 153.7, 164.7, 167.3 (×2);
PATENT
WO2011115173
https://patentscope.wipo.int/search/en/detail.jsf;jsessionid=CB6FAC725A85FC9DDE6D08A63CD4B038.wapp1nB?docId=WO2011115173&tab=FULLTEXT&queryString=ALL%3A%28%25E7%2582%258E%25E7%2597%2587%25E6%2580%25A7%25E8%2585%25B8%25E7%2596%25BE%25E6%2582%25A3%29&recNum=236&maxRec=6346


Example 1-1 Production of 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin- Production of On (1a) (Manufacture of Free Body)
[Chemical Formula 5]
a) 65 g (359 mmol) of 7-hydroxy-3,4-dihydro-2H-1,4-benzothiazin-3-one obtained by the method described in JP-A-60-4176 and JP-A-59-70675, Was suspended in tetrahydrofuran (194 mL) under an argon atmosphere, 104 g (397 mmol) of triphenylphosphine and 32 mL (379 mmol) of 3-chloropropanol were added and the mixture was cooled to 0 ° C. Next, 78 mL (396 mmol) of azodicarboxylic acid diisopropyl ester was added dropwise to the obtained reaction solution at 30 ° C. or less, and the mixture was stirred at room temperature for 1 hour. The solvent was distilled off from the resulting solution under reduced pressure, methanol (390 mL) was added thereto, and the mixture was stirred at room temperature for 1 hour. The precipitated crystals were collected by filtration and then dried under reduced pressure at 50 ° C. for 5 hours to obtain 59 g (yield 64%) of 7- (3-chloropropoxy) -3,4-dihydro-2H-1,4-benzothiazin- ) As blue-white crystals.
[Chemical Formula 6]

1 H-NMR (400 MHz, DMSO-d 6 ) δ: 2.12 (2H, quint, J = 6.2 Hz), 3.28 (2H, s), 3.76 (2H, t, J = (2H, t, J = 5.8 Hz), 6.78 (1 H, dd, J = 2.8, 8.8 Hz), 6.88 (1 H, d, J = 8.8 Hz ), 6.90 (1 H, d, J = 2.8 Hz), 10.38 (1 H, s)
57 g (221 mmol) of 7- (3-chloropropoxy) -3,4-dihydro-2H-1,4-benzothiazin-3-one was suspended in dimethylformamide (172 mL), 49 g (355 mmol) of potassium carbonate, 40 g (241 mmol) of potassium iodide and 43 g (231 mmol) of Nt-butoxycarbonylpiperazine were added and the mixture was heated to 100 ° C. and stirred for 4 hours. Water (344 mL) was added to the reaction solution, and the mixture was cooled to 0 ° C. and further stirred at the same temperature for 1 hour. The precipitated crystals were collected by filtration and then dried under reduced pressure at 50 ° C. for 5 hours to give 7- [3- (Nt-butoxycarbonylpiperazinyl) propoxy] -3,4-dihydro-2H-1,4-benzothiazine -3-one (89% yield) as bluish-white crystals.
[Chemical Formula 7]

1 H-NMR (400 MHz, DMSO-d 6 ) δ: 1.39 (9 H, s), 1.83 (2 H, quint, J = 6.8 Hz), 2.31 (4 H, t, J = 4. 3.30 (2H, t, J = 4.6 Hz), 3.41 (2H, s), 3.95 (2H, t, J = 6.4 Hz), 6.78 (1 H, dd, J = 2.8, 8.8 Hz), 6.88 (1 H, d, J = 8.8 Hz), 6.89 (1 H, s) 10.38 (1 H, s)
c) 87 g (214 mmol) of 7- {3- (Nt-butoxycarbonylpiperazinyl) propoxy} -3,4-dihydro-2H- 1,4-benzothiazin-3-one was suspended in ethanol (174 mL) , 6N hydrochloric acid aqueous solution (174 mL) was added dropwise at 50 ° C., and the mixture was stirred at the same temperature for 1 hour. Ethanol (522 mL) was added to the reaction solution, followed by cooling to 0 ° C. and further stirring at the same temperature for 1 hour. The precipitated crystals were collected by filtration and then dried under reduced pressure at 50 ° C. for 5 hours to give 7- {3- (piperazin-1-yl) propoxy} -3,4-dihydro-2H-1,4-benzothiazin- · Hydrochloride salt 75 g (yield 92%) was obtained as blue-white crystals.
[Chemical Formula 8]

1 H-NMR (400 MHz, D 2 O) [delta]: 2.13 (2H, td, J = 5.9,15.6Hz), 3.34 (2H, s), 3.35 (2H, t, J = 8.0 Hz), 3.44-3.64 (8H, m), 4.02 (2H, t, J = 5.6 Hz), 6.74 (1H, dd, J = 2.4, 6.85 (1 H, d, J = 8.8 Hz), 6.90 (1 H, d, J = 2.4 Hz)
d) 1- (2-ethoxyethyl) -2-chloromethyl-1H-benzimidazole obtained by the method described in Journal of Heterocyclic Chemistry (1987), 24 (1), 31-37 was dissolved in tetrahydrofuran (293 mL) and Was dissolved in a mixture of water (147 mL), and 7- {3- (Nt-butoxycarbonylpiperazinyl) propoxy} -3,4-dihydro-2H- 73 g (192 mmol) of 1,4-benzothiazin-3-one was added. Then, 117 mL (673 mmol) of diisopropylethylamine and 35 g (211 mmol) of potassium iodide were added, and the mixture was stirred at room temperature for 15 hours. Ethyl acetate (293 mL) and water (147 mL) were added to the reaction solution and extracted, and the organic layer was washed with 20% brine (147 mL). The organic layer was concentrated under reduced pressure to give 115 g (2 steps, quantitative) of the title compound (1a) as a brown oil.
1 H-NMR (400 MHz, CDCl 3 ) δ: 1.13 (3H, t, J = 7.0 Hz), 1.93 (2H, quint, J = 6.9 Hz), 2.40-2.70 (2H, s), 3.42 (2H, q, J = 6.8 Hz), 3.76 (2H, t, J = 7.2 Hz), 2.51 5. 2 (t, J = 6.0 Hz), 3.88 (2H, s), 3.97 (2H, t, J = 6.2 Hz), 4.51 (2H, t, J = 5.8 Hz), J = 8.8 Hz), 6.85 (1 H, d, J = 2.4 Hz), 7.24 (1 H, d, -7.28 (2H, m), 7.39 (1 H, ddd, J = 1.2, 6, 6.8 Hz), 7.73 (1 H, ddd, J = 1.2, 6.0 , 6.8 Hz) 8.35 (1H, s)
Example 1-2: 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H-1,4-benzothiazin- Production of On Monomaleate (2a) (Production of Seed Crystal)
[Chemical Formula 9]

7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin-3-one (1a) 0 g (1.96 mmol) was dissolved in ethanol (8 mL) and warmed to 60 ° C. After adding 211 mg (1.80 mmol) of maleic acid and stirring at 50 ° C. for 1 hour, the mixture was stirred at room temperature for 16 hours and further stirred at 0 ° C. for 3 hours. The precipitated crystals were collected by filtration and then dried under reduced pressure at 50 ° C. for 5 hours to obtain 1.02 g (yield 91%) of the monomaleate (2a) as bluish white crystals (melting point: 148 ° -151 ° C.).
Examples 1-3: 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin- Preparation of on-monomaleate (2a)
7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin-3-one (1a). After dissolving 0 g (13.7 mmol) in ethanol (56 mL) and heating to 60 ° C., 1.46 g (12.6 mmol) of maleic acid was added and the mixture was cooled to 50 ° C. to obtain 0.035 g (0.056 mmol) of seed crystals was added. The reaction solution was stirred at 50 ° C. for 1 hour, then stirred at room temperature for 1 hour, and further stirred at 0 ° C. for 3 hours. The precipitated crystals were collected by filtration and then dried under reduced pressure at 50 ° C. for 5 hours to obtain 7.08 g (yield 90%) of monomaleate (2a) as bluish-white crystals.
1 H-NMR (400 MHz, DMSO-d 6 ) δ: 1.02 (3H, t, J = 7.2 Hz), 2.00-2.07 (2H, m), 2.80-3.61 J = 5.2 Hz), 3.93 (2H, q, J = 6.9 Hz), 3.42 (2H, s), 3.71 (2H, (2H, t, J = 5.2 Hz), 6.03 (2H, s), 6.78 (1 H, dd, J = 2.4, 8.8 Hz), 6.88 (1 H, s), 6.91 (1 H, dd, J = 2.4, 2.4 Hz), 7.18 (1 H, ddd, J = 1 (2H, d, J = 8.4 Hz), 7.24 (1H, ddd, J = 1.4, 7.5, 7.5 Hz), 7.59 10.40 (1 H, s)
Elementary analysis value of the monomaleate (2a) obtained in Example 1-3: C 31 H 39 N 5 O 7 S
: theoretical value: C 59.50%; H 6.28%; N 11.19 %
Found: C 59.33%; H 6.29%; N 11.10%
7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazine obtained in Example 1-3 -3-one monomaleate (2a) was subjected to thermal analysis measurement. In the thermal analysis measurement, approximately 5 mg of a sample was accurately weighed in an aluminum pan for thermal analysis, Al 2 O 3 was used as a reference substance , and the temperature was raised at a heating rate of 10 ° C./min in the presence of an atmosphere of N 2 gas (150 mL / min) (DTA) and thermogravimetry (TG) using a Thermo Plus 2 system (manufactured by Rigaku) as a thermal analyzer. The results of the thermal analysis measurement are shown in FIG. The melting point of the monomaleate (2a) was 147-150 ° C. (B – 545, manufactured by BUCHI).
7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazine obtained in Example 1-3 -3-one monomaleate (2a) by infrared spectrophotometer (manufactured by Thermo Nicolet Co., Ltd., AVATAR 370; ATR method) shows the pattern shown in FIG. 2, and it is in the vicinity of 1669 cm -1 , 1492Cm -1 around, 1231Cm -1 around, 1208Cm -1around, 868Cm -1 and around 754Cm -1 had an absorption peak specific to the vicinity.
7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazine obtained in Example 1-3 -3-one monomaleate (2a) was measured by powder X-ray diffraction (Miniflex manufactured by Rigaku Denki Kogyo Co., Ltd.). Measurement of powder X-ray crystal diffraction was carried out by filling the sample in the sample holder part of the silicon non-reflecting sample plate for X-ray diffraction and measuring with a desktop X-ray diffractometer: MiniFlex (Rigaku) a scanning range of diffraction angle 2θ; 3.00 ° to 40.00 °, sampling width: 0.02 °, and scanning speed: 2.00 ° / min. The obtained diffraction pattern is shown in FIG. 3. The monomaleate (2a) had specific diffraction angles and relative intensities shown in Table 1
[table 1]

Examples 1-4: 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin-3- Preparation of On Monomaleate (2a) (Study of Reproducibility on Large Scale)
(1a) (115 g) was added to a solution of 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4-dihydro-2H-1,4-benzothiazin- 226 mmol) was dissolved in ethanol (293 mL), activated charcoal 5.5 g was added, and the mixture was stirred at room temperature for 1 hour, then filtered through celite and washed with ethanol (147 mL) and washed. Ethanol (147 mL) was added to the filtrate, and after heating to 60 ° C., 18.9 g (163 mmol) of maleic acid was added and cooled to 50 ° C. 0.58 g (0.93 mmol) of the seed crystals of the monomaleate (2a) obtained in Example 1-3 was added and stirred at 50 ° C. for 1 hour, followed by stirring at room temperature for 15 hours and further at 0 ° C. And the mixture was stirred for 3 hours. The precipitated crystals were collected by filtration and dried under reduced pressure at 50 ° C. for 5 hours to obtain 75.2 g (yield 63%) of monomaleate (2a) as white crystals (melting point: 147 ° -149 ° C.).
Elementary analysis value of the monomaleate (2a) obtained in Examples 1-4: C 31 H 39 N 5 O 7 S
: theoretical value: C 59.50%; H 6.28%; N 11.19 %
Found: C 59.41%; H 6.29%; N 11.08%
Comparative Example 1 Synthesis of 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin- Preparation of dimaleate
15. 9 g (31 (3-ethoxyethylbenzoimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4-dihydro-2H-1,4- benzothiazin- . 1 mmol) was dissolved in 70 mL of ethanol, the solution was heated to 60 ° C., 8.0 g (68.9 mmol) of maleic acid was added, and the mixture was stirred at room temperature for 15 hours. The precipitated crystals were collected by filtration and then dried under reduced pressure at 50 ° C. for 5 hours to give 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- 13.3 g of dihydro-2H-1,4-benzothiazin-3-one / dimaleate was obtained. The obtained compound was dissolved in methanol (13 mL), heated to 60 ° C., THF (52 mL) was added, and the mixture was stirred at room temperature for 20 hours. The obtained crystals were collected by filtration and dried under reduced pressure at 50 ° C. for 5 hours to give 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4 -Dihydro-2H-1,4-benzothiazin-3-one · dimaleate was obtained as blueish white crystals.
1 H-NMR (400 MHz, DMSO-d 6 ) δ: 1.01 (3H, t, J = 7.0 Hz), 2.00-2.07 (2H, m), 3.00 (4H, m) , 3.20 (2H, m), 3.37 (2H, q, J = 6.9 Hz), 3.41-3.47 (4H, m), 3.70 (2H, t, J = 5. (2H, t, J = 5.8 Hz), 4.50 (2H, t, J = 5.0 Hz), 6.14 (4H, s), 3.95 (2H, s) , 6.76 (1 H, dd, J = 2.4, 8.8 Hz), 6.88 (1 H, s), 6.90 (1 H, m), 7.19 – 7.27 (2 H, m) , 7.60 (2H, d, J = 7.6 Hz), 10.40 (1 H, s)
Comparative Example 2 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin-3-one Production of monofumarate
6.81 g of 13- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) – 1 – piperazinyl} propoxy] -3,4- dihydro-2H-1,4-benzothiazin- . 3 mmol) was dissolved in a mixed solvent of ethanol (60 mL) and (water 6 mL), and the mixture was heated to 60 ° C. To the mixed solution was added a mixed solution of ethanol (14 mL) containing 1.55 g (13.3 mmol) of fumaric acid and water (1.5 mL), the mixture was stirred at 40 ° C. for 30 minutes, and further stirred at room temperature for 20 hours . The precipitated crystals were collected by filtration and dried under reduced pressure at 40 ° C. for 53.5 hours to give 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] 6.16 g (yield: 74%) of 4-dihydro-2H-1,4-benzothiazin-3-one monofumarate was obtained as slightly yellow crystals.
1 H-NMR (400 MHz, DMSO-d 6 ) δ: 1.01 (3H, t, J = 7.0 Hz), 1.81 (2H, quint, J = 6.6 Hz), 2.40-2. J = 5.6 Hz), 3.78 (2H, s), 3.93 (2H, m), 3.72 (2H, J = 6.4 Hz), 4.47 (2H, t, J = 5.2 Hz), 6.60 (2H, s), 6.75 (1 H, dd, J = 3.0, 9.0 Hz , 6.87 (1 H, d, J = 8.8 Hz), 6.89 (1 H, s), 7.15 (1 H, t, J = 7.6 Hz), 7.20 (1 H, t, J = 7.4 Hz), 7.54 (2H, t, J = 7.6 Hz), 10.36 (1 H, s)
Comparative Example 3 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 1,4-benzothiazin-3-one Production of disulfate
8.28 g (16 parts) of 7- [3- {4- (N-ethoxyethylbenzoimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4-dihydro-2H- 1,4-benzothiazin- . 2 mmol) was dissolved in a mixed solvent of ethanol (104 mL) and water (11 mL) and cooled to 0 ° C. A solution of 3.19 g (16.2 mmol) of sulfuric acid in water (11 mL) was added dropwise and the mixture was stirred at 40 ° C. for 30 minutes, and further stirred at room temperature for 20 hours. The precipitated crystals were collected by filtration and dried under reduced pressure at 40 ° C. for 53.5 hours to give 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] (86% yield) of 4-dihydro-2H-1,4-benzothiazin-3-one disulfate as slightly yellow crystals.
1 H-NMR (400 MHz, DMSO-d 6 ) δ: 1.02 (3H, t, J = 6.8 Hz), 2.03 (2H, m), 2.65 (2H, m), 3.00 (4H, m), 3.26 (2H, m), 3.37 (2H, q, J = 6.8 Hz), 3.41-3.47 (4H, m), 3.75 , J = 5.0 Hz), 4.01 (2H, t, J = 5.8 Hz), 4.21 (2H, brs), 4.65 (2H, t, J = 5.0 Hz), 6.78 J = 8.8 Hz), 6.90 (1 H, d, J = 3.2 Hz), 7.50 – (1 H, d, J = 2.8, 9.2 Hz), 6.89 7.55 (2H, m), 7.79 (1H, d, J = 8.4 Hz), 7.91 (1H, d, J = 6.0 Hz), 10.41 (1H, s)
Presence or Absence of Crystallization of Each Product]
The monomaleate (2a) obtained in Example 1-3 and the comparative compound obtained in Comparative Examples 1 to 3 (the dimaleate of the title compound (1a) , Monofumarate, disulfate) were obtained as crystals as described above. On the other hand, salts of hydrochloric acid, boric acid, phosphoric acid and citric acid were prepared as a comparative example using the title compound (1a) in the same manner as in Comparative Example 2, and crystallization of each compound was attempted. Upon crystallization of each product, methanol or ethanol was used as a crystallization solvent. The results are shown in Table 2.
[Table 2]
Crystallization studies gave crystalline salts for sulfuric acid, hydrochloric acid, maleic acid and fumaric acid. On the other hand, the borate, phosphate and citrate of the title compound (1a) did not crystallize, the monoborate was an oily substance and the monophosphate and the monocitrate were amorphous. For the maleate, hydrochloride and sulfate of the title compound (1a), a double salt was obtained in addition to the 1-fold salt. The hydrochloride salt of the title compound (1a) showed clear deliquescence for both monohydrochloride salt and dihydrochloride salt.
[Comparison of Purification Efficiency of Monomeric Acid Salt and Dimaleate Salt of
Title Compound (1a) ] Monomaleate and dimaleate of the title compound (1a) were synthesized under the same conditions using the same means to give crystals Was obtained. Means of synthesis of each product is shown below.
(A) Synthesis of
Monomeric Salt of Title Compound (1a) 7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- -1,4-benzothiazin-3-one (1a) (8.26 g, 16.2 mmol) was added to 71.74 g of ethanol and heated to 60 ° C., 1.79 g (15.40 mmol) of maleic acid was added , Cooled to 50 ° C. and 40 mg (0.064 mmol) of seed crystals was added. The reaction solution was stirred at 50 ° C. for 1 hour and then stirred overnight at room temperature. Subsequently, the reaction solution was stirred at 3 ° C. or less for 5 hours. After completion of the stirring, the precipitated crystals were collected by filtration to obtain 6.26 g (yield 62%) of the monomaleic acid salt of the title compound (1a).
(B) Synthesis of Dimaleate of Title Compound (1a)
7- [3- {4- (N-ethoxyethylbenzimidazol-2-ylmethyl) -1-piperazinyl} propoxy] -3,4- dihydro-2H- 8.26 g (16.2 mmol) of 1,4-benzothiazin-3-one (1a) was added to 71.74 g of ethanol and heated to 60 ° C., and 4.7 g (40.48 mmol) of maleic acid was added. After confirming that the maleic acid was completely dissolved in the solution, it was stirred overnight at room temperature. Subsequently, the reaction solution was stirred at 3 ° C. or less for 5 hours. After completion of the stirring, the precipitated crystals were collected by filtration to obtain 8.04 g (yield 67%) of the dimaleic acid salt of the title compound (1a).
[0114]
Crystals of the monomaleate and dimaleate obtained by means (a) and (b) above were each dissolved in a small amount of solvent and the purity of each substance was measured by high performance liquid chromatography (HPLC). The HPLC conditions are as follows and charts showing the HPLC measurement results are shown in FIGS. 4 and 5. Table 3 summarizes the HPLC measurement results.
Column: A stainless steel tube having an inner diameter of 4.6 mm and a length of 5 cm was
charged
with 3.5 μm of phenylhexylsilylated silica gel for liquid chromatography (HPLC) .
( B%) 20% → <10 minutes> → 60% (10 minutes) → <10 minutes>
Column temperature: constant temperature around 40 ° C.
Gradient condition (B%) 20% → 85% (10 min)
A solution: 0.01 mol / L phosphate buffer, pH 6.0
B: methanol
flow rate: 1.0 mL / min
area measurement range: 40 minutes
injection volume: 3 [mu] L
sample concentration: 1 mg / mL
PATENT
JP 2013035773
JP 2013049632
1.(a) Fukuda, T.; Koketsu, A.; Kaneko, Y.; Ashikawa, Y. Monomaleate of Benzothiazine Compound. WO2011115173, 2011.
(b) Fukuda, T.; Koketsu, A. Method for Producing Benzothiazine Compound. WO2011115150, 2011.
For monoalkylation of piperazine, see:
(a) Foye, W. O.; Levine, H. B.; McKenzie, W. L. α-(N-Piperazino)dimethylacetanilides and Their Local Anesthetic Activity. J. Med. Chem. 1966,9, 61– 63, DOI: 10.1021/jm00319a016
.
(b) Wang, T.; Zhang, Z.; Meanwell, N. A. Regioselective Monobenzoylation of Unsymmetrical Piperazines. J. Org. Chem. 2000, 65, 4740– 4742, DOI: 10.1021/jo000005e
.
(c) Guillaume, M.; Cuypers, J.; Vervest, I.; De Smaele, D.; Leurs, S. Synthesis of T2288: From Bench Synthesis to Pilot Production. Org. Process Res. Dev. 2003, 7, 939– 941, DOI: 10.1021/op034117u
.
(d) Aalla, S.; Gilla, G.; Anumula, R. R.; Kurella, S.; Padi, P. R.; Vummenthala, P. R. Improved Process for Ranolazine: An Antianginal Agent. Org. Process Res. Dev. 2012, 16, 748– 754, DOI: 10.1021/op300026r
(b) Fukuda, T.; Koketsu, A. Method for Producing Benzothiazine Compound. WO2011115150, 2011.
//////////K-8986, K 8986,
O=C(O)/C=C\C(=O)O.CCOCCn4c5ccccc5nc4CN1CCN(CC1)CCCOc2ccc3NC(=O)CSc3c2
























