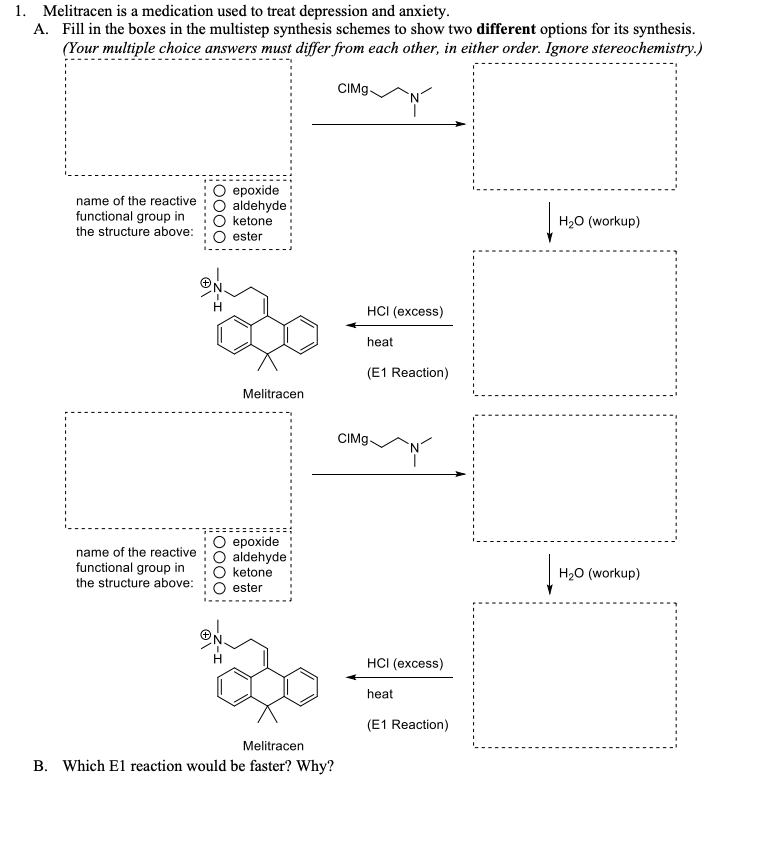
Melitracen
- Molecular FormulaC21H25N
- Average mass291.430 Da
Melitracen (brand names Melixeran) is a tricyclic antidepressant (TCA), for the treatment of depression and anxiety.[1][2][3][4] In addition to single drug preparations, it is also available as Deanxit, marketed by Lundbeck, a combination product containing both melitracen and flupentixol.[5][6][7][8]
The pharmacology of melitracen has not been properly investigated and is largely unknown, but it is likely to act in a similar manner to other TCAs. Indeed, melitracen is reported to have imipramine and amitriptyline-like effects and efficacy against depression and anxiety, though with improved tolerability and a somewhat faster onset of action.[9][10]
- ATC:N06AA14
- MW:291.44 g/mol
- CAS-RN:5118-29-6
- InChI Key:GWWLWDURRGNSRS-UHFFFAOYSA-N
- InChI:InChI=1S/C21H25N/c1-21(2)19-13-7-5-10-17(19)16(12-9-15-22(3)4)18-11-6-8-14-20(18)21/h5-8,10-14H,9,15H2,1-4H3
- EINECS:225-858-5
- LD50:52 mg/kg (M, i.v.); 315 mg/kg (M, p.o.);
170 mg/kg (R, p.o.)
Derivatives
hydrochloride
- Formula:C21H25N • HCl
- MW:327.90 g/mol
- CAS-RN:10563-70-9
- EINECS:234-150-5
- LD50:52 mg/kg (M, i.v.); 315 mg/kg (M, p.o.);
170 mg/kg (R, p.o.)
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 90-44-8 | C14H10O | anthrone | 9(10H)-Anthracenone |
| 85118-29-2 | C21H27NO | 9-[3-(dimethylamino)propyl]-9,10-dihydro-10,10-dimethyl-9-anthracenol | 9-Anthracenol, 9-[3-(dimethylamino)propyl]-9,10-dihydro-10,10-dimethyl- |
| 19070-16-7 | C5H12ClMgN | 3-dimethylaminopropylmagnesium chloride | Magnesium, chloro[3-(dimethylamino)propyl]- |
| 5447-86-9 | C16H14O | 10,10-dimethylanthrone | 9(10H)-Anthracenone, 10,10-dimethyl- |
SYN

 |
||
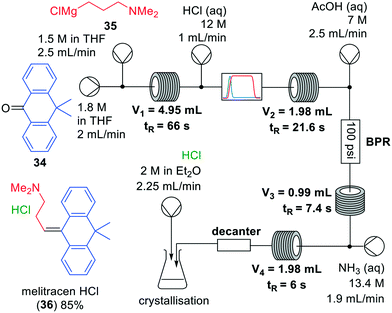
| Fig. 10 Synthesis of melitracen HCl-(36) by Kiil and co-workers making use of a one-flow system. Adapted with permission from Org. Process Res. Dev., 2018, 22, 228–235. Copyright 2018 American Chemical Society.35 | ||
Grignard reactions are commonly used for the construction of carbon–carbon bonds and show exothermic behaviour which can be dangerous in large-scale batch processes. The use of Grignard reagents in flow can be beneficial because of the high control of reaction conditions, facile heat transport and small effective reaction volume.6,34 A recent example was published by Kiil and co-workers, who synthesised melitracen (36) in a one-flow system.35 Kiil hypothesised that the seven unit operations required in batch could be decreased by combining a hydrolysis and dehydration step, and removing a phase separation (Fig. 10).
The investigation commenced with finding a suitable solvent for the Grignard reaction in which starting materials 34, 35 and intermediate products would dissolve. After having identified THF as the most suitable option, the next challenge was to find an acid that could induce both hydrolysis and dehydration in a single step. Hydrochloric acid was able to perform both transformations, however, precipitation was observed. Thus, hydrochloric acid molarities ranging from 1–12 M were tested. However, while even at the lowest molarity precipitation was observed, it also appeared that below 6 M the dehydration reaction did not proceed. Since the precipitation could not be prevented, a molarity of 12 M was eventually used. The individually optimised transformations were then combined in a one-flow continuous system. Most troublesome was that addition of HCl to the reaction mixture led to an exothermic reaction and boiling of the solvent. Therefore, a back-pressure regulator was employed so that melitracen (36) could be successfully synthesised as its HCl-salt in approximately 85% yield.
SYN
https://pubs.acs.org/doi/pdf/10.1021/acs.oprd.7b00368
A Grignard-based batch process, for the preparation of Melitracen HCl, has been redesigned to fit a continuous reactor system. The Grignard addition is carried out at room temperature, with subsequent hydrolysis of the magnesium alkoxide intermediate followed by dehydration of the resulting alcohol. The product undergoes further workup by simple gravimetric phase separation and then crystallization with 2 M HCl in diethyl ether to afford pure Melitracen HCl. All steps in the laboratory setup were concatenated, and the setup was proven capable of producing a significant portion of the commercial quantities of Melitracen HCl. The flow setup profits from a reduced footprint, lower energy consumption, fewer synthetic steps, and reduced raw material usage compared to the batch process.

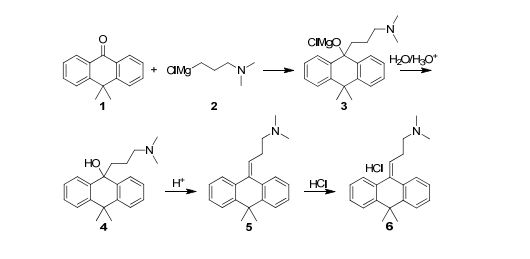
As illustrated in Scheme 1, four synthetic steps are involved in the manufacturing of Melitracen HCl (6). The four steps are a classic Grignard addition to a ketone, a hydrolysis of a magnesium alkoxide, a dehydration of an alcohol and a salt precipitation to isolate the API. The Grignard addition is between 10,10-dimethylanthrone (10,10-DMA (1)) and 3-(N,N-dimethylamino)propylmagnesium chloride (DMPC-MgCl (2)), resulting in formation of the magnesium alkoxide 3. The magnesium alkoxide 3 is then hydrolyzed to the alcohol 4 and dehydrated to form product 5. The last step is a crystallization of the API as a salt, where HCl is added to obtain the Melitracen HCl (6)
Scheme 1: Syntheses of magnesium alkoxide 3, alcohol 4 and dehydrated product 5 in the manufacturing process of Melitracen HCl 6, from ketone 1 and Grignard reagent 2.
Current Batch Synthesis The current batch synthesis involves individual synthetic steps, as illustrated in Figure 1. DMPC-MgCl 2 is made in-house before it is used, due to its limited storage shelf life, in a toluene-THF solvent mixture. THF is present in trace amounts in order to stabilize the magnesium in the Grignard reagents.45 A solution of 10,10-DMA 1 is prepared in toluene and is slowly transferred to the DMPC-MgCl 2, maintaining a temperature of 50°C. DMPC-MgCl 2 is used in an equivalence of 1.6 compared to 10,10-DMA 1. The formed magnesium alkoxide 3 is hydrolyzed with water and acetic acid (80%). The aqueous phase is discarded and concentrated hydrochloric acid (37%) is used to dehydrate alcohol 4 to form dehydrated product 5. Toluene is replaced with ethanol by a solvent swap. Crystallization of the dehydrated product 5 from the ethanol phase is done with HCl gas to obtain the final Melitracen HCl (6), which is subsequently isolated by filtration.
Precipitation of Melitracen HCl from THF The dehydrated product 5 was crystallized as the final HCl salt in the THF in a batch experiment, in order to remove a solvent swap to ethanol. The crystallization was carried out with 2 M HCl in Et2O, as this was considered more suited for a later flow process and more easily implemented in the laboratory setup. An equivalence of 1.1 HCl was used and the requirement was an achievement of pH<2. The mixture was kept stirred during the crystallization and carried out at ambient temperature. After 10 minutes, fine white solids started to form, followed by a massive precipitation of Melitracen HCl 6. The Melitracen HCl 6 was filtered with a Büchner funnel and washed with THF. The isolated yield was 80% and within the specifications for the in-house analysis methods used in the routine production (CHN, TGA, UV-vis, HPLC, melting point). Figure 3 is a microscope picture of the isolated Melitracen HCl 6. For full-scale production, the HCl gas would still be more desirable for the crystallization and the 2 M HCl in Et2O merely serves as a proof of concept for the laboratory flow setup.
CLIP
PATENT
https://patents.google.com/patent/CN105418436B/en

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
References
- ^ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ^ Hall, Chapman and; Chemical Abstracts Service, American Chemical Society; Rhodes, P. H (1996). Dictionary of organic compounds. London: Chapman & Hall. ISBN 0-412-54090-8.
- ^ O’Neil, Maryadele J. (2001). The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Rahway, NJ: Merck Research Laboratories. ISBN 0-911910-13-1.
- ^ José Miguel Vela; Helmut Buschmann; Jörg Holenz; Antonio Párraga; Antoni Torrens (2007). Antidepressants, Antipsychotics, Anxiolytics: From Chemistry and Pharmacology to Clinical Application. Weinheim: Wiley-VCH. ISBN 978-3-527-31058-6.
- ^ Muller, Niels F; Dessing, Rudolf P; Pharmacy, European Society of Clinical (1998). European Drug Index, 4th Edition. Boca Raton: CRC Press. ISBN 3-7692-2114-1.
- ^ Van Moffaert M, Dierick M, De Meulemeester F, Vereecken A (1983). “Treatment of depressive anxiety states associated with psychosomatic symptoms. A double-blind multicentre clinical study: mianserin versus melitracen-flupentixol”. Acta Psychiatrica Belgica. 83 (5): 525–39. PMID 6670581.
- ^ Bin Yaacob H (April 1985). “Flupenthixol and Melitracen in the management of trigeminal neuralgia”. Dental Journal of Malaysia. 8 (2): 37–8. PMID 3917005.
- ^ Hashash JG, Abdul-Baki H, Azar C, et al. (June 2008). “Clinical trial: a randomized controlled cross-over study of flupenthixol + melitracen in functional dyspepsia”. Alimentary Pharmacology & Therapeutics. 27 (11): 1148–55. doi:10.1111/j.1365-2036.2008.03677.x. PMID 18331614. S2CID 40714136.
- ^ Aronson, Jeffrey Kenneth (2008). Meyler’s Side Effects of Psychiatric Drugs (Meylers Side Effects). Amsterdam: Elsevier Science. ISBN 978-0-444-53266-4.
- ^ Author Unknown (1970). Ann Reports Medicinal Chem V5 (v. 5). Boston: Academic Press. ISBN 0-12-040505-9.
{{cite book}}:|author=has generic name (help)
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Adaptol, Dixeran, Melixeran, Thymeol, Trausabun |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral, intramuscular injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.023.507 |
| Chemical and physical data | |
| Formula | C21H25N |
| Molar mass | 291.438 g·mol−1 |
| 3D model (JSmol) | |
| |
|
//////////Melitracen, Q7T0Y1109Z, Thymeol, мелитрацен , ميليتراسان , 美利曲辛 , U 24973A, Antidepressant, Tricyclics,