Metopimazine
RP-9965, EXP-999, NG-101
l-(3-[2-(methylsulfonyl)-10H-phenothiazin-10-yl]propyl)-4-piperidinecarboxamide
- Isonipecotamide, 1-[3-[2-(methylsulfonyl)phenothiazin-10-yl]propyl]- (7CI,8CI)
- 1-[3-[2-(Methylsulfonyl)-10H-phenothiazin-10-yl]propyl]-4-piperidinecarboxamide
- 1-[3-[2-(Methylsulfonyl)phenothiazin-10-yl]propyl]-4-piperidinecarboxamide
- 1-[3-[2-(Methylsulfonyl)phenothiazin-10-yl]propyl]isonipecotamide
- 2-Methylsulfonyl-10-[3-(4-carbamoylpiperidino)propyl]phenothiazine
- EXP 999
- Metopimazine
- RP 9965
- Vogalene
- metopimazine (gastroparesis), Neurogastrx
Sanofi (Originator)
Teva
Treatment of Nausea and Vomiting, APPROVED
Dopamine D3 receptor antagonist; Dopamine D2 receptor antagonist
Gastroprokinetic
Metopimazine (INN) is a phenothiazine antiemetic.
Metopimazine is an established antiemetic that has been approved and marketed for many years in Europe for the treatment of acute conditions. The compound does not cross the blood-brain-barrier, and is therefore free from central side effects, and is not associated with cardiovascular side effects
In May 2016, preclinical data were presented at the 2016 DDW in San Diego, CA. In rats, po NG-101 and domperidone did not penetrate the brain at therapeutically relevant concentrations, unlike metoclopramide. In dogs, the amplitude and frequency of antral contractions were increased by NG-101, whereas in rats, po metopimazine resulted in an increase in gastric emptying of solid foods. The blood-brain barrier was not readily crossed and there was no interaction with 5-HT3 or 5-HT4 receptors by NG-101 unlike metoclopramide and domperidone, respectively
Neurogastrx is investigating repurposed metopimazine (NG-101), a selective and peripherally restricted dopamine D2/D3 receptor antagonist, for the potential oral treatment of gastroparesis. By July 2014, preclinical studies were underway . SE BELOW REF
WO-2014105655: Methods for treating GI tract disorders
In May 2016, preclinical data were presented [SEE BELOW].
2016 May 24Abs 1079
NG101: A Potent and Selective Dopamine D2 Receptor Antagonist as a Potential Alternative to Metoclopramide and Domperidone for the Treatment of Gastroparesis
Digestive Disease Week
Cyril De Colle, Marieke van der Hart, Jiande Chen, Arash Rassoulpour, Pankaj J Pasricha
In July 2014, preclinical data were published. Metopimazine at 1mg/kg increased gastric motility in hound dogs. In studies in rodents, metopimazine at 3 and 10 mg/kg increased gastric emptying by 18 and 40%, respectively, compared with vehicle control
There is an increasing demand for antiemetic agents because of the most troublesome adverse effects of chemotherapy-induced nausea and emesis during cancer treatment.
However, the objective of complete prevention of emesis in all patients remains elusive. Therefore, there is a great demand for both development of (i) new antiemetic agents and (ii) new manufacturing processes for existing antiemetic agents. Metopimazine is an existing dopamine D2-receptor antagonist with potent antiemetic properties. It is chemically known as l-(3-[2-(methylsulfonyl)-10H-phenothiazin-10-yl]propyl)-4-piperidinecarboxamide , which belongs to nitrogen- and sulfur-containing tricyclic compounds (phenothiazine class of drugs) with interesting biological and pharmacological activities.
Recently, it has been found that Metopimazine plays a key role as an alternative to Ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderate to high emetogenic noncisplatin-based chemotherapy.It has been used in France for many years for the prevention and treatment of nausea and vomiting under the brand name of Vogalene
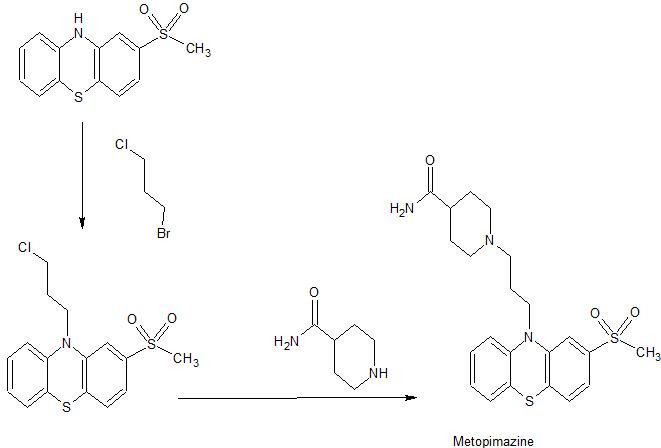
In 1959, the first synthesis and manufacture process of Metopimazine was reported by Jacob et al.The synthesis starts from the protection of 2-(Methylsulfanyl)-10H-phenothiazine..Jacob, R. M.; Robert, J. G. German Patent No. DE1092476, 1959.
Later, in 1990, Sindelar et al. reported a modified process , which starts from synthesis of 4-(2-fluorophenylthio)-3-nitrophenylmethylsulfone..Sindelar, K.; Holubek, J.; Koruna, I.; Hrubantova, M.; Protiva, M. Collect. Czech. Chem. Commun. 1990, 55, 1586– 1601, DOI: 10.1135/cccc19901586
In 2010, Satyanarayana Reddy et al. reported a modified synthetic route which starts from either N-protection using acetyl chloride or N-alkylation using dihalopropane of 2-(methylsulfanyl)-10H-phenothiazine ..Satyanarayana Reddy, M.; Eswaraiah, S.; Satyanarayana, K. Indian Patent No. 360/CHE/2010 A, Aug 19, 2011.
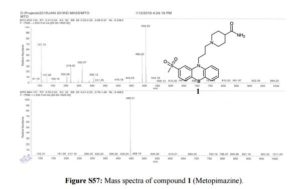
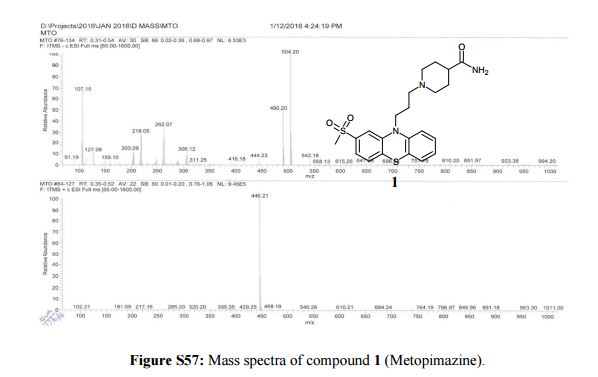
Synthesis of 1-(3-[2-(methylsulfonyl)-10H-phenothiazin-10-yl]propyl)piperidine-4- carboxamide (1)-Metopimazine: Pale yellow color solid, yield. 65% (82 g), DSC 189 °C.
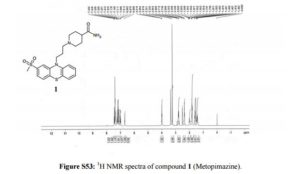
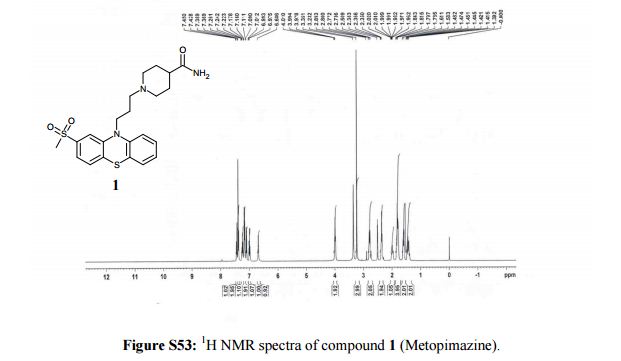
1H NMR (400 MHz, DMSO-d6, δ/ppm): 7.44 (d, 1H, arom H, J = 8.8 Hz), 7.37 (d, 2H, arom H, J = 8.0 Hz), 7.24 (t, 1H, arom H, J = 7.6 Hz), 7.16 (m, 2H, -NH2), 7.1 (d, 1H, arom H, J = 8.4 Hz), 6.99 (t, 1H, arom H, J = 7.6 Hz), 6.68 (s, 1H, arom H), 3.99 (t, 2H, -NCH2, J = 6.4 Hz), 3.23 (s, 3H, -S-CH3), 2.8-2.73 (m, 2H, -CH2-), 2.36 (t, 2H, -CH2-, J = 6.8 Hz), 2.02-1.96 (m, 1H, -CH-), 1.84-1.78 (m, 4H, 2-CH2-), 1.61-1.58 (m, 2H, -CH2-), 1.48-1.44 (m, 2H, – CH2-).
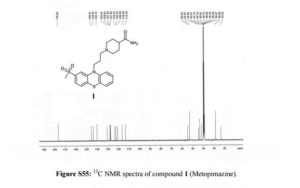
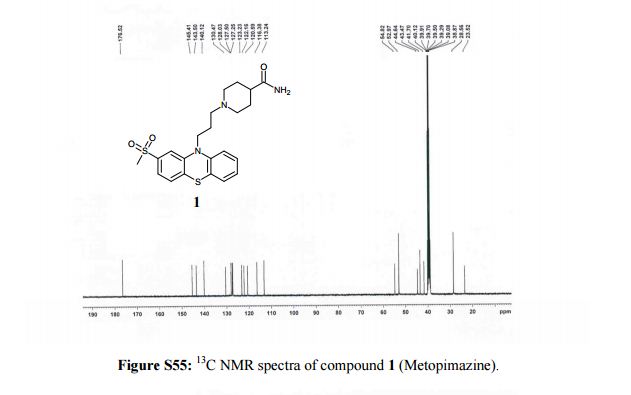
13C NMR (100 MHz, DMSO-d6, δ/ppm): 176.52, 145.41, 143.5, 140.12, 130.47, 128.03, 127.50, 127.25, 123.23, 122.16, 120.59, 116.38, 113.24, 54.82, 52.97, 44.64, 43.47, 41.7, 28.59, 23.52.
MS m/z (ESI): 446.21 (M+H)+.
SYNTHESIS

PATENT
IN 201641043070
IN 2013CH05689
IN 2013CH00361
IN 2010CH00360
DE 1092476/US 3130194
PAPER
A Simple and Commercially Viable Process for Improved Yields of Metopimazine, a Dopamine D2-Receptor Antagonist
http://pubs.acs.org/doi/abs/10.1021/acs.oprd.7b00052

An efficient, practical, and commercially viable manufacturing process was developed with ≥99.7% purity and 31% overall yield (including four chemical reactions and one recrystallization) for an active pharmaceutical ingredient, called Metopimazine (1), an antiemetic drug used to prevent emesis during chemotherapy. The development of two in situ, one-pot methods in the present synthetic route helped to improve the overall yield of 1 (31%) compared with earlier reports (<15%). For the first time, characterization data of API (1), intermediates, and also possible impurities are presented. The key process issues and challenges were addressed effectively and achieved successfully.
Synthesis of 1-(3-[2-(Methylsulfonyl)-10H-phenothiazin-10-yl]propyl)-4-piperidinecarboxamide (1), Metopimazine
- Vogalene
- metopimazina (Italian, Portuguese)
- metopimazin (Danish, Swedish)
- metopimazine (Dutch)
- metopimatsiini (Finnish)
Regulatory List Number
- EC No.: 237-818-4
- EINECS No.: 237-818-4
-
Harmonized Tariff Code
293430
- Moda, Tiago L.; Bioorganic & Medicinal Chemistry 2007, 15(24), PG7738-7745
- “Drugs – Synonyms and Properties” data were obtained from Ashgate Publishing Co. (US)
- Julou, Louis; Comptes Rendus des Seances de l’Academie des Sciences, Serie D: Sciences Naturelles 1968, 266(25), PG 2365-8
- Bounoure, Frederic; Journal of Inclusion Phenomena and Macrocyclic Chemistry 2007, 57(1-4), PG191-195
- Sindelar, Karel; Collection of Czechoslovak Chemical Communications 1990, 55(6), PG1586-601
- “PhysProp” data were obtained from Syracuse Research Corporation of Syracuse, New York (US)
- Habibi-Yangjeh, Aziz; Bulletin of the Korean Chemical Society 2008, 29(4), PG833-841
- Metwally, Fadia H.; Bulletin of the Faculty of Pharmacy (Cairo University) 2006, 44(3), PG1-15
- WO-2014105655: Methods for treating GI tract disorders
- 2016 May 24Abs 1079
NG101: A Potent and Selective Dopamine D2 Receptor Antagonist as a Potential Alternative to Metoclopramide and Domperidone for the Treatment of Gastroparesis
Digestive Disease Week
Cyril De Colle, Marieke van der Hart, Jiande Chen, Arash Rassoulpour, Pankaj J Pasricha
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.034.367 |
| Chemical and physical data | |
| Formula | C22H27N3O3S2 |
| Molar mass | 445.6 g/mol |
| 3D model (Jmol) | |
//////////////Metopimazine, Dopamine D2-Receptor Antagonist, 14008-44-7, sanofi, teva, RP-9965, Nausea and Vomiting, EXP-999, NG-101, metopimazine, gastroparesis, Neurogastrx
NC(=O)C1CCN(CC1)CCCN2c4ccccc4Sc3ccc(cc23)S(C)(=O)=O