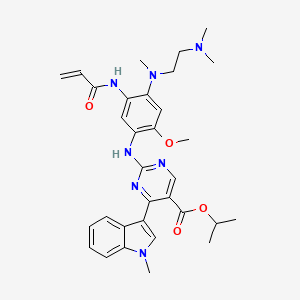
Mobocertinib
1847461-43-1
MF C32H39N7O4
MW 585.70
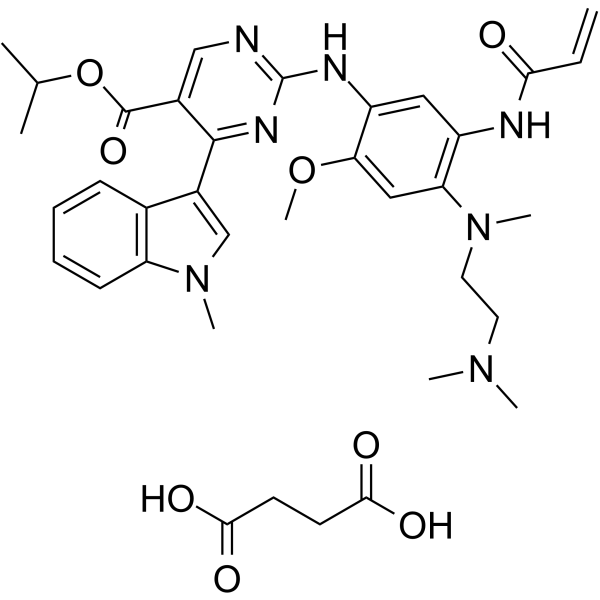
propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
TAK-788, AP32788, TAK788, UNII-39HBQ4A67L, AP-32788, 39HBQ4A67L
US10227342, Example 10, MFCD32669806, NSC825519, s6813, TAK-788;AP32788, WHO 11183
NSC-825519, example 94 [WO2015195228A1], GTPL10468, BDBM368374, BCP31045, EX-A3392
US FDA APPROVED 9/15/2021, Exkivity, To treat locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutation
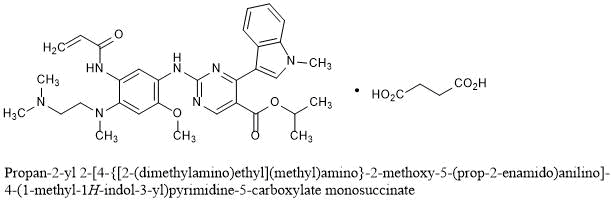
Mobocertinib succinate Chemical Structure
CAS No. : 2389149-74-8
| Molecular Weight |
703.78 |
|---|---|
| Formula |
C₃₆H₄₅N₇O₈ |
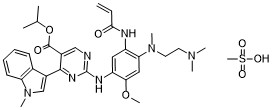
Molecular Weight: 681.809
CAS #: 2389149-85-1 (mesylate) 1847461-43-1 (free base) 2389149-74-8 (succinate) 2389149-76-0 (HBr) 2389149-79-3 (HCl) 2389149-81-7 (sulfate) 2389149-83-9 (tosylate) 2389149-87-3 (oxalate) 2389149-89-5 (fumarate)
JAPANESE ACCEPTED NAME
Mobocertinib Succinate
Propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate monosuccinate
C32H39N7O4▪C4H6O4 : 703.78
[2389149-74-8]
FDA grants accelerated approval to mobocertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations……. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20
On September 15, 2021, the Food and Drug Administration granted accelerated approval to mobocertinib (Exkivity, Takeda Pharmaceuticals, Inc.) for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
Today, the FDA also approved the Oncomine Dx Target Test (Life Technologies Corporation) as a companion diagnostic device to select patients with the above mutations for mobocertinib treatment.
Approval was based on Study 101, an international, non-randomized, open-label, multicohort clinical trial (NCT02716116) which included patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations. Efficacy was evaluated in 114 patients whose disease had progressed on or after platinum-based chemotherapy. Patients received mobocertinib 160 mg orally daily until disease progression or intolerable toxicity.
The main efficacy outcome measures were overall response rate (ORR) according to RECIST 1.1 as evaluated by blinded independent central review (BICR) and response duration. The ORR was 28% (95% CI: 20%, 37%) with a median response duration of 17.5 months (95% CI: 7.4, 20.3).
The most common adverse reactions (>20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain. Product labeling includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
The recommended mobocertinib dose is 160 mg orally once daily until disease progression or unacceptable toxicity.
View full prescribing information for mobocertinib.
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
This review was conducted under Project Orbis, an initiative of the FDA Oncology Center of Excellence. Project Orbis provides a framework for concurrent submission and review of oncology drugs among international partners. For this review, FDA collaborated with the Australian Therapeutic Goods Administration (TGA), the Brazilian Health Regulatory Agency (ANVISA), and United Kingdom’s Medicines & Healthcare products Regulatory Agency (MHRA). The application reviews are ongoing at the other regulatory agencies.
This review used the Assessment Aid, a voluntary submission from the applicant to facilitate the FDA’s assessment. The FDA approved this application approximately 6 weeks ahead of the FDA goal date.
This application was granted priority review, breakthrough therapy designation and orphan drug designation. A description of FDA expedited programs is in the Guidance for Industry: Expedited Programs for Serious Conditions-Drugs and Biologics.
- Approval based on Phase 1/2 trial results, which demonstrated clinically meaningful responses with a median duration of response (DoR) of approximately 1.5 years
- Next-generation sequencing (NGS) companion diagnostic test approved simultaneously to support identification of patients with EGFR Exon20 insertion mutations
OSAKA, Japan, and CAMBRIDGE, Mass. September 15, 2021 – Takeda Pharmaceutical Company Limited (TSE:4502/NYSE:TAK) (“Takeda”) today announced that the U.S. Food and Drug Administration (FDA) has approved EXKIVITY (mobocertinib) for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy. EXKIVITY, which was granted priority review and received Breakthrough Therapy Designation, Fast Track Designation and Orphan Drug Designation from the FDA, is the first and only approved oral therapy specifically designed to target EGFR Exon20 insertion mutations. This indication is approved under Accelerated Approval based on overall response rate (ORR) and DoR. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
“The approval of EXKIVITY introduces a new and effective treatment option for patients with EGFR Exon20 insertion+ NSCLC, fulfilling an urgent need for this difficult-to-treat cancer,” said Teresa Bitetti, president, Global Oncology Business Unit, Takeda. “EXKIVITY is the first and only oral therapy specifically designed to target EGFR Exon20 insertions, and we are particularly encouraged by the duration of the responses observed with a median of approximately 1.5 years. This approval milestone reinforces our commitment to meeting the needs of underserved patient populations within the oncology community.”
The FDA simultaneously approved Thermo Fisher Scientific’s Oncomine Dx Target Test as an NGS companion diagnostic for EXKIVITY to identify NSCLC patients with EGFR Exon20 insertions. NGS testing is critical for these patients, as it can enable more accurate diagnoses compared to polymerase chain reaction (PCR) testing, which detects less than 50% of EGFR Exon20 insertions.
“EGFR Exon20 insertion+ NSCLC is an underserved cancer that we have been unable to target effectively with traditional EGFR TKIs,” said Pasi A. Jänne, MD, PhD, Dana Farber Cancer Institute. “The approval of EXKIVITY (mobocertinib) marks another important step forward that provides physicians and their patients with a new targeted oral therapy specifically designed for this patient population that has shown clinically meaningful and sustained responses.”
“Patients with EGFR Exon20 insertion+ NSCLC have historically faced a unique set of challenges living with a very rare lung cancer that is not only underdiagnosed, but also lacking targeted treatment options that can improve response rates,” said Marcia Horn, executive director, Exon 20 Group at ICAN, International Cancer Advocacy Network. “As a patient advocate working with EGFR Exon20 insertion+ NSCLC patients and their families every day for nearly five years, I am thrilled to witness continued progress in the fight against this devastating disease and am grateful for the patients, families, healthcare professionals and scientists across the globe who contributed to the approval of this promising targeted therapy.”
The FDA approval is based on results from the platinum-pretreated population in the Phase 1/2 trial of EXKIVITY, which consisted of 114 patients with EGFR Exon20 insertion+ NSCLC who received prior platinum-based therapy and were treated at the 160 mg dose. Results were presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting from the Phase 1/2 trial and demonstrated a confirmed ORR of 28% per independent review committee (IRC) (35% per investigator) as well as a median DoR of 17.5 months per IRC, a median overall survival (OS) of 24 months and a median progression-free survival (PFS) of 7.3 months per IRC.
The most common adverse reactions (>20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain. The EXKIVITY Prescribing Information includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings and precautions for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
The FDA review was conducted under Project Orbis, an initiative of the FDA Oncology Center of Excellence (OCE), which provides a framework for concurrent submission and review of oncology products among international partners. We look forward to continuing our work with regulatory agencies across the globe to bring mobocertinib to patients.
About EXKIVITY (mobocertinib)
EXKIVITY is a first-in-class, oral tyrosine kinase inhibitor (TKI) specifically designed to selectively target epidermal growth factor receptor (EGFR) Exon20 insertion mutations.
EXKIVITY is approved in the U.S. for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
Results from the Phase 1/2 trial of mobocertinib have also been accepted for review by the Center for Drug Evaluation (CDE) in China for locally advanced or metastatic NSCLC patients with EGFR Exon20 insertion mutations who have been previously treated with at least one prior systemic chemotherapy.
For more information about EXKIVITY, visit www.EXKIVITY.com. For the Prescribing Information, including the Boxed Warning, please visit https://takeda.info/Exkivity-Prescribing-Information.
About EGFR Exon20 Insertion+ NSCLC
Non-small cell lung cancer (NSCLC) is the most common form of lung cancer, accounting for approximately 85% of the estimated 2.2 million new cases of lung cancer diagnosed each year worldwide, according to the World Health Organization.1,2 Patients with epidermal growth factor receptor (EGFR) Exon20 insertion+ NSCLC make up approximately 1-2% of patients with NSCLC, and the disease is more common in Asian populations compared to Western populations.3-7 This disease carries a worse prognosis than other EGFR mutations, as EGFR TKIs – which do not specifically target EGFR Exon20 insertions – and chemotherapy provide limited benefit for these patients.
Takeda is committed to continuing research and development to meet the needs of the lung cancer community through the discovery and delivery of transformative medicines.
EXKIVITY IMPORTANT SAFETY INFORMATION
QTc Interval Prolongation and Torsades de Pointes: EXKIVITY can cause life-threatening heart rate-corrected QT (QTc) prolongation, including Torsades de Pointes, which can be fatal, and requires monitoring of QTc and electrolytes at baseline and periodically during treatment. Increase monitoring frequency in patients with risk factors for QTc prolongation. Avoid use of concomitant drugs which are known to prolong the QTc interval and use of strong or moderate CYP3A inhibitors with EXKIVITY, which may further prolong the QTc. Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity of QTc prolongation.
Interstitial Lung Disease (ILD)/Pneumonitis: Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis. Immediately withhold EXKIVITY in patients with suspected ILD/pneumonitis and permanently discontinue EXKIVITY if ILD/pneumonitis is confirmed.
Cardiac Toxicity: Monitor cardiac function, including left ventricular ejection fraction, at baseline and during treatment. Withhold, resume at reduced dose or permanently discontinue based on severity.
Diarrhea: Diarrhea may lead to dehydration or electrolyte imbalance, with or without renal impairment. Monitor electrolytes and advise patients to start an antidiarrheal agent at first episode of diarrhea and to increase fluid and electrolyte intake. Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity.
Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective non-hormonal contraception.
Mobocertinib, sold under the brand name Exkivity, is used for the treatment of non-small cell lung cancer.[2][3]
The most common side effects include diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain.[2]
Mobocertinib is a small molecule tyrosine kinase inhibitor. Its molecular target is epidermal growth factor receptor (EGFR) bearing mutations in the exon 20 region.[4][5]
Mobocertinib was approved for medical use in the United States in September 2021.[2][3] It is a first-in-class oral treatment to target EGFR Exon20 insertion mutations.[3]
Medical uses
Mobocertinib is indicated for adults with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.[2]

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter a
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
PATENT
WO 2019222093
https://patents.google.com/patent/WO2019222093A1

PATENT
WO 2015195228
https://patents.google.com/patent/WO2015195228A1/en
PATENT
https://patents.google.com/patent/US10227342
| isopropyl 2-((5-acrylamido-4-((2- | R13 | |
| (dimethylamino)ethyl)(methyl)amino)-2- | ||
| methoxyphenyl)amino)-4-(1-methyl-1H- | ||
| indol-3-yl)pyrimidine-5-carboxylate | ||
| 1H NMR (CDCl3) δ 10.15 (s, 1 H), 9.80 | ||
| (s, 1 H), 8.91 (s, 1 H), 8.70 (br. s., 1 H), | ||
| 7.91 (s, 1 H), 7.48-7.71 (m, 1 H), 7.15- | ||
| 7.37 (m, 3 H), 6.81 (s, 1 H), 6.49 (dd, | ||
| J = 17.07, 1.88 Hz, 1 H), 6.36 (dd, | ||
| J = 16.94, 10.04 Hz, 1 H), 5.73 (dd, | ||
| J = 10.04, 1.88 Hz, 1 H), 5.02 (dt, | ||
| J = 12.45, 6.26 Hz, 1 H), 4.00 (s, 3 H), | ||
| 3.90 (s, 3 H), 2.86-2.93 (m, 2 H), 2.76 | ||
| (s, 3 H), 2.26-2.31 (m, 8 H), 1.05 (d, | ||
| J = 6.15 Hz, 6 H) | ||
| ESI-MS m/z: 586.3 [M + H]+ |
References
- ^ Jump up to:a b https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215310s000lbl.pdf
- ^ Jump up to:a b c d e “FDA grants accelerated approval to mobocertinib for metastatic non-sma”. U.S. Food and Drug Administration (FDA). 16 September 2021. Retrieved 16 September 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b c “Takeda’s Exkivity (mobocertinib) Approved by U.S. FDA as the First Oral Therapy Specifically Designed for Patients with EGFR Exon20 Insertion+ NSCLC” (Press release). Takeda Pharmaceutical Company. 15 September 2021. Retrieved 16 September 2021 – via Business Wire.
- ^ “TAK-788 as First-line Treatment Versus Platinum-Based Chemotherapy for Non-Small Cell Lung Cancer (NSCLC) With EGFR Exon 20 Insertion Mutations”. Clinicaltrials.gov. Retrieved 17 February 2021.
- ^ Zhang SS, Zhu VW (2021). “Spotlight on Mobocertinib (TAK-788) in NSCLC with EGFR Exon 20 Insertion Mutations”. Lung Cancer. Auckland, N.Z. 12: 61–65. doi:10.2147/LCTT.S307321. PMC 8286072. PMID 34285620.
External links
- “Mobocertinib”. Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02716116 for “A Study of TAK-788 in Adults With Non-Small Cell Lung Cancer” at ClinicalTrials.gov
 |
|
| Clinical data | |
|---|---|
| Trade names | Exkivity |
| Other names | TAK-788 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth |
| Drug class | Antineoplastic |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C32H39N7O4 |
| Molar mass | 585.709 g·mol−1 |
| 3D model (JSmol) | |
////////////mobocertinib, Exkivity, TAK 788, AP32788, fda 2021, approvals 2021, cancer
CC(C)OC(=O)C1=CN=C(N=C1C2=CN(C3=CC=CC=C32)C)NC4=C(C=C(C(=C4)NC(=O)C=C)N(C)CCN(C)C)OC