OTERACIL
UNII4R7FFA00RX, CAS Number2207-75-2, WeightAverage: 195.175, Monoisotopic: 194.96823705, Chemical FormulaC4H2KN3O4
[K+].OC1=NC(=NC(=O)N1)C([O-])=O
-
KOX
-
NSC 28841
-
Oxonate
-
Oxonate, potassium
CDSCO APPROVED,01.02.2022

Gimeracil bulk & Oteracil potassium bulk and Tegafur 15mg/20mg, Gimeracil 4.35mg/5.8mg and Oteracil 11.8mg/15.8mg capsules
indicated in adults for the treatment of advanced gastric cancer when given in combination with cisplatin.
Oteracil Potassium is the potassium salt of oxonate, an enzyme inhibitor that modulates 5- fluorouracil (5-FU) toxicity. Potassium oxonate inhibits orotate phosphoribosyltransferase, which catalyzes the conversion of 5-FU to its active or phosphorylated form, FUMP. Upon oral administration, Oxonate is selectively distributed to the intracellular sites of tissues lining the small intestines, producing localized inhibitory effects within the gastrointestinal tract. As a result, 5-FU associated gastrointestinal toxic effects are reduced and the incidence of diarrhea or mucositis is decreased in 5-FU related therapy.
Oteracil is an adjunct to antineoplastic therapy, used to reduce the toxic side effects associated with chemotherapy. Approved by the European Medicines Agency (EMA) in March 2011, Oteracil is available in combination with Gimeracil and Tegafur within the commercially available product “Teysuno”. The main active ingredient in Teysuno is Tegafur, a pro-drug of Fluorouracil (5-FU), which is a cytotoxic anti-metabolite drug that acts on rapidly dividing cancer cells. By mimicking a class of compounds called “pyrimidines” that are essential components of RNA and DNA, 5-FU is able to insert itself into strands of DNA and RNA, thereby halting the replication process necessary for continued cancer growth.
Oteracil’s main role within Teysuno is to reduce the activity of 5-FU within normal gastrointestinal mucosa, and therefore reduce’s gastrointestinal toxicity 1. It functions by blocking the enzyme orotate phosphoribosyltransferase (OPRT), which is involved in the production of 5-FU.
/////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
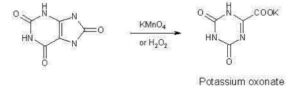
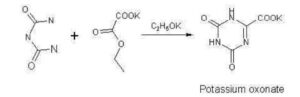
SYNTHESIS
https://patents.google.com/patent/CN103435566A/zh
SYN
https://europepmc.org/article/pmc/pmc7717319
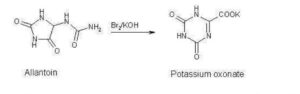
Poje et al. reported a two-step, gram-scale preparation of the TS-1 additive oteracil 21 (Scheme 16).226 Iodine-mediated-oxidation of uric acid 116 produced dehydroallantoin 117 as the major product, and subsequent treatment with potassium hydroxide resulted in the rearranged product oteracil 21.227

Synthesis of Oteracil 21a
aReagents and conditions: (a) LiOH, I2, H2O, 5 °C, 5 min, then AcOH, 75%; (b) aq KOH, 20 min, rt, 82%.
//////////OTERACIL POTTASIUM, KOX, NSC 28841, Oxonate, Oxonate potassium, INDIA 2022, APPROVALS 2022, CANCER
[K+].OC1=NC(=NC(=O)N1)C([O-])=O