Photobiocatalytic alcohol oxidation using LED light sources
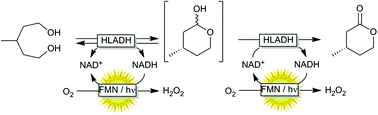
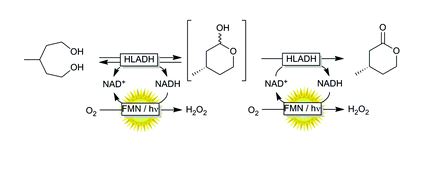
Oxidative lactonization of meso-3-methyl-1,5-pentanediol to (S)-4-methyltetrahydro-2H-pyran-2-one using horse liver alcohol dehydrogenase (HLADH) and photocatalytic, aerobic regeneration of NAD+.
DOI: 10.1039/C6GC02008A, Communication
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
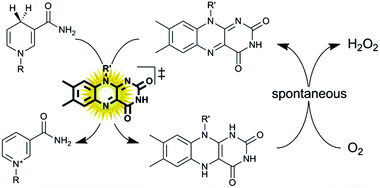
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.The photocatalytic oxidation of NADH using a flavin photocatalyst and a simple blue LED light source is reported.
Photobiocatalytic alcohol oxidation using LED light sources
E-mail: f.hollmann@tudelft.nl
DOI: 10.1039/C6GC02008A, http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C6GC02008A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract

The photocatalytic oxidation of NADH using a flavin photocatalyst and a simple blue LED light source is reported. This in situ NAD+ regeneration system can be used to promote biocatalytic, enantioselective oxidation reactions. Compared to the traditional use of white light bulbs this method enables very significant reductions in energy consumption and CO2 emission.
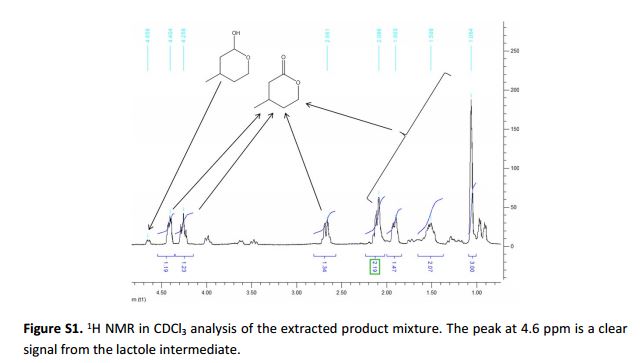
Synthesis of (S)-4-methyltetrahydro-2H-pyran-2-one catalyzed by HLADH The synthesis of (S)-4-methyltetrahydro-2H-pyran-2-one was performed as previously reported by Kara et al. (2013).[3] For this, a stock of meso-3-methyl-1,5-pentanediol (0.5 M), NAD+ stock (25 mM), acetosyringone stock (2 mM), and HLADH stock (3 gL–1 ) were freshly prepared in 50 mM Tris-HCl buffer at pH 8. The laccase was applied as delivered (0.2 mM solution). The mixture of meso-3- methyl-1,5-pentanediol stock (1 mL), acetosyringone stock (1 mL), NAD+ stock (0.2 mL) and buffer (6.7 mL) was incubated at 30 °C for 5 min. Finally, laccase (0.1 mL) and HLADH solution (1 mL) were added. The starting concentrations were: 50 mM meso-3-methyl-1,5-pentanediol, 0.5 mM NAD+ , 200 µM acetosyringone, 0.3 gL–1 HLADH and 2 µM laccase. The reaction mixture (10 mL) was orbitaly shaken at 600 rpm in 50 mL Falcon tubes at 30 °C. Samples (50 µL) were taken at defined time intervals and mixed with 200 µL EtOAc (containing 5 mM acetophenone). The mixture was vortexed and dried over anhydrous MgSO4. A conversion of 72 % to the enantiopure (S)-4-methyltetrahydro- 2H-pyran-2-one (ee > 99% according to GC analysis) was achieved after 16 hours. The reaction mixture (10 mL) was then saturated with NaCl and extracted with EtOAc (3 x 10 mL). After each extraction step the mixture was centrifuged (4000 rpm, 10 min). The collected clear organic phase was dried over anhydrous MgSO4 and the solvent was removed under reduced pressure to give a yellowish oily compound (39 mg). Purification of the crude product was attempted by column chromatography (Pasteur pipette filled with Silica gel 60, 70-230 mesh particle size; solvent petroleum ether: ethyl acetate 9:1).
. Picture of the reaction setup. Commercially available LED bands (3 colored) were wrapped around a thermostatted reaction vessel and used for illumination of the reaction mixture inside (S)-4-Me-DVL (R)-4-Me-DVL (generally in a Schlenk vessel) a slight overpressure was achieved by an air-filled balloon to reduce O2-transfer limitations to the reaction mixture
//////////Photobiocatalytic alcohol oxidation, LED light sources