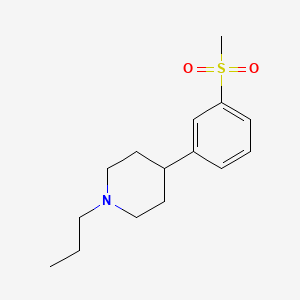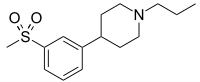
Pridopidine
- Molecular Formula C15H23NO2S
- Average mass 281.414 Da
| 4-[3-(Methylsulfonyl)phenyl]-1-propylpiperidine |
| ACR 16 |
|
| FR 310826 |
Huntingtons chorea
Dopamine D2 receptor antagonist; Opioid receptor sigma agonist 1
Neurosearch INNOVATORS, In 2012, the product was acquired by Teva
In January 2017, pridopidine was reported to be in phase 3 clinical development, pridopidine for treating or improving cognitive functions and Alzheimer’s disease.
Teva Pharmaceutical Industries, following an asset acquisition from NeuroSearch, is developing pridopidine, a fast-off dopamine D2 receptor antagonist that strengthens glutamate function, for treating HD.
The drug holds orphan drug designation in the U.S. and the E.U. for the treatment of Huntington’s disease
About Huntington Disease
HD is a fatal neurodegenerative disease for which there is no known cure or prevention. People who suffer from HD will likely have a variety of steadily-worsening symptoms, including uncoordinated and uncontrolled movements, cognition and memory deterioration and a range of behavioral and psychological problems. HD symptoms typically start in middle age, but the disease may also manifest itself in childhood and in old age. Disease progression is characterized by a gradual decline in motor control, cognition and mental stability, and generally results in death within 15 to 25 years of clinical diagnosis. Current treatment is limited to managing the symptoms of HD, as there are no treatments that have been shown to alter the progression of HD. Studies estimate that HD affects about 13 to 15 people per 100,000 in Caucasians, and for every affected person there are approximately three to five people who may carry the mutation but are not yet ill.

Pridopidine, also known as ACR16, is a dopamine stabilizer, which improves motor performance and shows neuroprotective effects in Huntington disease R6/2 mouse model. Huntington disease (HD) is a neurodegenerative disorder for which new treatments are urgently needed. Pridopidine is a new dopaminergic stabilizer, recently developed for the treatment of motor symptoms associated with HD.

Dopamine D2 ligands. Dopamine D2 receptor agonists dopamine (1) and apomorphine (2), classical antagonists haloperidol (3) and olanzapine (4), partial agonists (−)-3-(3-hydroxyphenyl)-N–n-propylpiperidine (5), bifeprunox (6), aripiprazole (7), and 3-(1-benzylpiperidin-4-yl)phenol (9a), and dopaminergic stabilizers S-(−)-OSU6162 (8) and pridopidine (12b).
Dopamine is a neurotransmitter in the brain. Since this discovery, made in the 1950s, the function of dopa-mine in the brain has been intensely explored. To date, it is well established that dopamine is essential in several aspects of brain function including motor, cognitive, sensory, emotional and autonomous (e.g. regulation of appetite, body temperature, sleep) functions. Thus, modulation of dopaminergic function may be beneficial in the treatment of a wide range of disorders affecting brain functions. In fact, both neurologic and psychiatric disorders are treated with medications based on interactions with dopamine systems and dopamine receptors in the brain.
Drugs that act, directly or indirectly, at central dopamine receptors are commonly used in the treatment of neurologic and psychiatric disorders, e.g. Parkinson’s disease and schizophrenia. Currently available dopaminer-gic pharmaceuticals have severe side effects, such as ex-trapyramidal side effects and tardive dyskinesia in dopaminergic antagonists used as antipsychotic agents, and dyskinesias and psychoses in dopaminergic agonists used as anti -Parkinson ‘ s agents. Therapeutic effects are un-satisfactory in many respects. To improve efficacy and reduce side effects of dopaminergic pharmaceuticals, novel dopamine receptor ligands with selectivity at specific dopamine receptor subtypes or regional selectivity are sought for. In this context, also partial dopamine receptor agonists, i.e. dopamine receptor ligands with some but not full intrinsic activity at dopamine receptors, are being developed to achieve an optimal degree of stimulation at dopamine receptors, avoiding excessive do-pamine receptor blockade or excessive stimulation.
Compounds belonging to the class of substituted 4- (phenyl-N-alkyl) -piperazine and substituted 4-(phenyl-N-alkyl) -piperidines have been previously reported. Among these compounds, some are inactive in the CNS, some dis-play serotonergic or mixed serotonergic/dopaminergic pharmacological profiles while some are full or partial dopamine receptor agonists or antagonists with high affinity for dopamine receptors.
A number of 4-phenylpiperazines and 4 -phenyl -piperidine derivatives are known and described, for example Costall et al . European J. Pharm. 31, 94, (1975), Mewshaw et al . Bioorg. Med. Chem. Lett., 8, 295, (1998). The reported compounds are substituted 4 -phenyl -piperazine ‘ s, most of them being 2-, 3- or 4 -OH phenyl substituted and displaying DA autoreceptor agonist properties .
Fuller R. W. et al , J. Pharmacol. Exp . Therapeut . 218, 636, (1981) disclose substituted piperazines (e.g. 1- (m-trifluoro-methylphenyl) piperazine) which reportedly act as serotonin agonists and inhibit serotonin uptake.
Fuller R. W. et al , Res. Commun. Chem. Pathol . Pharmacol. 17, 551, (1977) disclose the comparative effects on the 3 , 4-dihydroxy-phenylacetic acid and Res. Commun. Chem. Pathol. Pharmacol. 29, 201, (1980) disclose the compara-tive effects on the 5-hydroxyindole acetic acid concentration in rat brain by 1- (p-chlorophenol) -piperazine .
Boissier J. et al Chem Abstr. 61:10691c, disclose disubstituted piperazines. The compounds are reportedly adrenolytics, antihypertensives , potentiators of barbitu-rates, and depressants of the central nervous system.
A number of different substituted piperazines have been published as ligands at 5-HT1A receptors, for example Glennon R.A. et al J. Med. Chem., 31, 1968, (1988), van Steen B.J., J. Med. Chem., 36, 2751, (1993), Mokrosz, J. et al, Arch. Pharm. (Weinheim) 328, 143-148 (1995), and Dukat M.-L., J. Med. Chem., 39, 4017, (1996). Glennon R. A. discloses, in international patent applications WO93/00313 and WO 91/09594 various amines, among them substituted piperazines, as sigma receptor ligands. Clinical studies investigating the properties of sigma receptor ligands in schizophrenic patients have not generated evi-dence of antipsychotic activity, or activity in any other CNS disorder. Two of the most extensively studied selective sigma receptor antagonists, BW234U (rimcazole) and BMY14802, have both failed in clinical studies in schizophrenic patients (Borison et al , 1991, Psychopharmacol Bull 27(2): 103-106; Gewirtz et al , 1994, Neuropsycho-pharmacology 10:37-40) .
Further, WO 93/04684 and GB 2027703 also describe specific substituted piperazines useful in the treatment of CNS disorders
Pridopidine (Huntexil, formerly ACR16) is an experimental drug candidate belonging to a class of agents known as dopidines, which act as dopaminergic stabilizers in the central nervous system. These compounds may counteract the effects of excessive or insufficient dopaminergic transmission,[1][2] and are therefore under investigation for application in neurological and psychiatric disorders characterized by altered dopaminergic transmission, such as Huntington’s disease (HD).
Pridopidine is in late-stage development by Teva Pharmaceutical Industries who acquired the rights to the product from its original developer NeuroSearch in 2012. In April 2010, NeuroSearch announced results from the largest European phase 3 study in HD carried out to date (MermaiHD). The MermaiHD study examined the effects of pridopidine in patients with HD and the results showed after six months of treatment, pridopidine improved total motor symptoms, although the primary endpoint of the study was not met. Pridopidine was well tolerated and had an adverse event profile similar to placebo.[3]
The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have both indicated they will not issue approval for pridopidine to be used in human patients on the basis of the MermaiHD and HART trials, and a further, positive phase 3 trial is required for approval.[4][5]

Dopidines
Dopidines, a new class of pharmaceutical compounds, act as dopaminergic stabilizers, enhancing or counteracting dopaminergic effects in the central nervous system.[1][2] They have a dual mechanism of action, displaying functional antagonism of subcortical dopamine type 2 (D2) receptors, as well as strengthening of cortical glutamate and dopamine transmission.[6] Dopidines are, therefore, able to regulate both hypoactive and hyperactive functioning in areas of the brain that receive dopaminergic input (i.e. cortical and subcortical regions). This potential ability to restore the cortical–subcortical circuitry to normal suggests dopidines may have the potential to improve symptoms associated with several neurological and psychiatric disorders, including HD.
SYNTHESIS

aReagents and conditions: (a) n-butyllithium, 1-Boc-4-piperidone, THF; (b) trifluoroacetic acid, CH2Cl2, Δ; (c) triethylamine, methyl chloroformate, CH2Cl2; (d) m-CPBA, CH2Cl2; (e) Pd/C, H2, MeOH, HCl; (f) HCl, EtOH, Δ; (g) RX, K2CO3, acetonitrile, Δ.
Pharmacology
In vitro studies demonstrate pridopidine exerts its effects by functional antagonism of D2 receptors. However, pridopidine possesses a number of characteristics[1][2][6][7] that differentiate it from traditional D2 receptor antagonists (agents that block receptor responses).
- Lower affinity for D2 receptors than traditional D2 ligands[8]
- Preferential binding to activated D2 (D2high) receptors (i.e. dopamine-bound D2 receptors)[8]
- Rapid dissociation (fast ‘off-rate’) from D2 receptors
- D2 receptor antagonism that is surmountable by dopamine
- Rapid recovery of D2-receptor-mediated responses after washout[1][2][6][7]
Pridopidine is less likely to produce extrapyramidal symptoms, such as akinesia (inability to initiate movement) and akathisia (inability to remain motionless), than dopamine antagonists (such as antipsychotics).[9] Furthermore, pridopidine displays no detectable intrinsic activity,[9][10] differentiating it from D2 receptor agonists and partial agonists (agents that stimulate receptor responses). Pridopidine, therefore, differs from D2 receptor antagonists, agonists and partial agonists.[6]
As a dopaminergic stabilizer, pridopidine can be considered to be a dual-acting agent, displaying functional antagonism of subcortical dopaminergic transmission and strengthening of cortical glutamate transmission.
- In vivo, pridopidine interacts with D2 receptors[9] and increases striatal levels of the dopamine metabolite 3,4-dihydroxyphenylacetic acid.[6]
- In vivo, pridopidine increases cortical expression of the gene encoding activity-regulated cytoskeletal protein (Arc)[6] and reverses perturbed behavioural patterns in hypoglutamatergic states (MK-801-induced hyperactivity models).[6][11][12][13]
Clinical development
The MermaiHD study
In 2009, NeuroSearch completed the largest European HD trial to date, the Multinational EuRopean Multicentre ACR16 study In Huntington’s Disease (MermaiHD) study.
This six-month, phase 3, randomized, double-blind, placebo-controlled trial recruited patients from Austria, Belgium, France, Germany, Italy, Portugal, Spain and the UK, and compared two different pridopidine dose regimens with placebo. Patients were randomly allocated to receive pridopidine (45 mg once daily or 45 mg twice daily) or placebo. During weeks 1–4, patients received once-daily treatment (as a morning dose). Thereafter, patients took two doses (one morning and one afternoon dose) until the end of the treatment period. The study had a target recruitment of 420 patients; recruitment was finalized in April 2009 with 437 patients enrolled.[14]
The purpose of the study was to assess the effects of pridopidine on a specific subset of HD motor symptoms defined in the modified motor score (mMS).[14] The mMS comprises 10 items relating to voluntary motor function from the Unified Huntington’s Disease Rating Scale Total Motor Score (UHDRS—TMS).[14] Other study endpoints included the UHDRS—TMS, submotor items, cognitive function, behaviour and symptoms of depression and anxiety.
After six months of treatment, patients who received pridopidine 45 mg twice daily showed significant improvements in motor function, as measured by the UHDRS-TMS, compared with placebo. For the mMS, which was the primary endpoint of the study, a strong trend in treatment effect was seen, although statistical significance was not reached. Pridopidine was also very well tolerated, had an adverse event profile similar to placebo and gave no indication of treatment-associated worsening of symptoms.[3]
The MermaiHD study – open-label extension
Patients who completed the six-month, randomized phase of the MermaiHD study could choose to enter the MermaiHD open-label extension study and receive pridopidine 45 mg twice daily for six months. In total, 357 patients were enrolled into the MermaiHD open-label extension study and of these, 305 patients completed the entire 12-month treatment period.[15]
The objective of this study was to evaluate the long-term safety and tolerability profile of pridopidine and to collect efficacy data after a 12-month treatment period to support the safety evaluation. Safety and tolerability assessments included the incidence and severity of adverse events, routine laboratory parameters, vital signs and electrocardiogram measurements.[15]
Results from the MermaiHD open-label extension study showed treatment with pridopidine for up to 12 months (up to 45 mg twice daily for the first six months; 45 mg twice daily for the last six months) was well tolerated and demonstrated a good safety profile.[3][15]
The HART study
In October 2010, NeuroSearch reported results from their three-month, phase 2b, randomized, double-blind, placebo-controlled study carried out in Canada and the USA – Huntington’s disease ACR16 Randomized Trial (HART). This study was conducted in 28 centres and enrolled a total of 227 patients, who were randomly allocated to receive pridopidine 10 mg, 22.5 mg or 45 mg twice daily) or placebo.[14][16] During weeks 1–4, patients received once-daily treatment (as a morning dose). Thereafter, patients took two treatment doses (one morning and one afternoon dose) until the end of the treatment period. Study endpoints were the same as those for the MermaiHD study.
Results from the HART study were consistent with findings from the larger MermaiHD study. After 12 weeks of treatment with pridopidine 45 mg twice daily, total motor function significantly improved, as measured by the UHDRS–TMS. The primary endpoint, improvement in the mMS, was not met.[16]
In both studies, the effects on the UHDRS–TMS and the mMS were driven by significant improvements in motor symptoms such as gait and balance, and hand movements, deemed by the authors to be “clinically relevant”. However, the magnitude of the improvements was small. Pridopdiine demonstrated a favourable tolerability and safety profile, including no observations of treatment-related disadvantages in terms of worsening of other disease signs or symptoms.[15][16]
Compassionate use programme and open-ended, open-label study
To meet requests from patients and healthcare professionals for continued treatment with pridopidine, NeuroSearch has established a compassionate use programme in Europe to ensure continued access to pridopidine for patients who have completed treatment in the MermaiHD open-label extension study. The programme is active in all of the eight European countries where the MermaiHD study was conducted.
NeuroSearch has initiated an open-ended, open-label clinical study in the USA and Canada, called the Open HART study. In this study, all patients who have completed treatment in the HART study are offered the chance to restart treatment with pridopidine until either marketing approval has been obtained in the countries in question, or the drug’s development is discontinued. The first patients were enrolled in March 2011.[3]
Regulatory agency advice
The results of the MermaiHD and HART trials were presented to the American and European regulatory agencies: the FDA in March 2011 and EMA in May, 2011. Both agencies indicated insufficient evidence had been produced to allow approval in human patients, and a further phase 3 trial would be required for approval.[4][5]
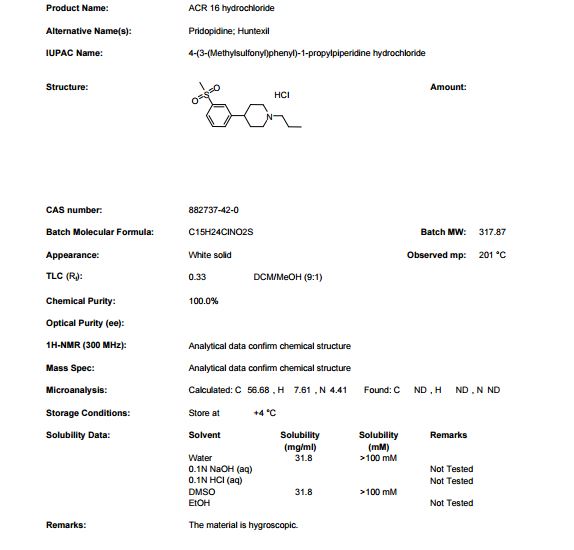
PATENT

Example 6: 4- (3 -Methanesulfonyl-phenyl ) – 1-propyl -piperidine
m.p. 200°C (HCl) MS m/z (relative intensity, 70 eV) 281 (M+, 5), 252 (bp) , 129 (20), 115 (20), 70 (25.
PAPER
Journal of Medicinal Chemistry (2010), 53(6), 2510-2520.
Synthesis and Evaluation of a Set of 4-Phenylpiperidines and 4-Phenylpiperazines as D2 Receptor Ligands and the Discovery of the Dopaminergic Stabilizer 4-[3-(Methylsulfonyl)phenyl]-1-propylpiperidine (Huntexil, Pridopidine, ACR16)
Abstract

Modification of the partial dopamine type 2 receptor (D2) agonist 3-(1-benzylpiperidin-4-yl)phenol (9a) generated a series of novel functional D2 antagonists with fast-off kinetic properties. A representative of this series, pridopidine (4-[3-(methylsulfonyl)phenyl]-1-propylpiperidine; ACR16, 12b), bound competitively with low affinity to D2 in vitro, without displaying properties essential for interaction with D2 in the inactive state, thereby allowing receptors to rapidly regain responsiveness. In vivo, neurochemical effects of 12b were similar to those of D2 antagonists, and in a model of locomotor hyperactivity, 12b dose-dependently reduced activity. In contrast to classic D2 antagonists, 12b increased spontaneous locomotor activity in partly habituated animals. The “agonist-like” kinetic profile of 12b, combined with its lack of intrinsic activity, induces a functional state-dependent D2 antagonism that can vary with local, real-time dopamine concentration fluctuations around distinct receptor populations. These properties may contribute to its unique “dopaminergic stabilizer” characteristics, differentiating 12b from D2 antagonists and partial D2agonists.
4-[3-(Methylsulfonyl)phenyl]-1-propylpiperidine (12b)
PATENT
Pridopidine (Huntexil®) is a unique compound developed for the treatment of patients with motor symptoms associated with Huntington’s disease. The chemical name of pridopidine is 4-(3-(Methylsulfonyl)phenyl)-l-propylpiperidine, and its Chemical Registry Number is CAS 346688-38-8 (CSED:7971505, 2016). The Chemical Registry number of pridopidine hydrochloride is 882737-42-0 (CSID:25948790 2016). Processes of synthesis of pridopidine and a pharmaceutically acceptable salt thereof are disclosed in U.S. Patent No. 7,923,459. U.S. Patent No. 6,903,120 claims pridopidine for the treatment of Parkinson’s disease, dyskinesias, dystonias, Tourette’s disease, iatrogenic and non-iatrogenic psychoses and hallucinoses, mood and anxiety disorders, sleep disorder, autism spectrum disorder, ADHD, Huntington’s disease, age-related cognitive impairment, and disorders related to alcohol abuse and narcotic substance abuse.
US Patent Application Publication Nos. 20140378508 and 20150202302, describe methods of treatment with high doses of pridopidine and modified release formulations of pridopidine, respectively.
EXAMPLES
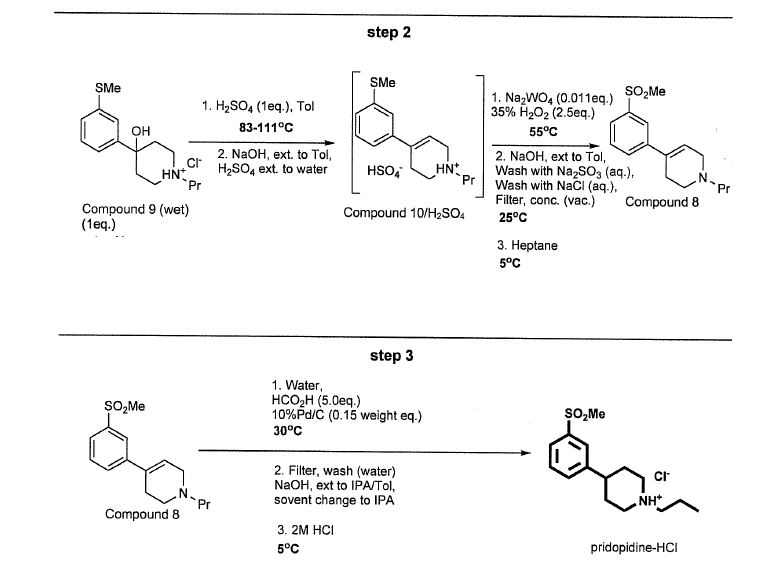
Example 1: Pridopidine-HCl synthesis
An initial process for synthesizing pridopidine HC1 shown in Scheme 1 and is a modification of the process disclosed in US Patent No. 7,923,459.
The synthesis of Compound 9 started with the halogen-lithium exchange of 3-bromothioanisole (3BTA) in THF employing n-hexyllithium (HexLi) in hexane as the lithium source. Li-thioanisole (3LTA) intermediate thus formed was coupled with 1 -propyl-4-piperidone (1P4P) forming a Li-Compound 9. These two reactions require low (cryogenic) temperature. The quenching of Li-Compound 9 was done in water HCl/MTBE resulting in precipitation of Compound 9-HCl salt. A cryogenic batch mode process for this step was developed and optimized. The 3BTA and THF were cooled to less than -70°C. A solution of HexLi in n-hexane (33%) was added at a temperature below -70°C and the reaction is stirred for more than 1 hour. An in-process control sample was taken and analyzed for completion of halogen exchange, l-propyl-4-piperidone (1P4P) was then added to the reaction at about -70°C letting the reaction mixture to reach -40°C and further stirred at this temperature for about 1 hour. An in-process sample was analyzed to monitor the conversion according to the acceptance criteria (Compound 9 not less than 83% purity). The reaction mixture was added to a mixture of 5N hydrochloric acid (HC1) and methyl teri-butyl ether (MTBE). The resulting precipitate was filtered and washed with MTBE to give the hydrochloric salt of Compound 9 (Compound 9-HCl) wet.
Batch mode technique for step 1 requires an expensive and high energy-consuming cryogenic system that cools the reactor with a methanol heat exchange, in which the methanol is circulated in counter current liquid nitrogen. This process also brings about additional problems originated from the workup procedure. The work-up starts when the reaction mixture is added into a mixture of MTBE and aqueous HC1. This gives three phases: (1) an organic phase that contains the organic solvents MTBE, THF and hexane along with other organic related materials such as thioanisole (TA), hexyl-bromide,
3-hexylthioanisole and other organic side reaction impurities (2) an aqueous phase containing inorganic salts (LiOH and LiBr), and (3) a solid phase which is mostly Compound 9-HCl but also remainders of 1P4P as an HC1 salt.
The isolation of Compound 9-HCl from the three phase work-up mixture is by filtration followed by MTBE washings. A major problem with this work-up is the difficulty of the filtration which resulted in a long filtration and washing operations. The time it takes to complete a centrifugation and washing cycle is by far beyond the normal duration of such a manufacturing operation. The second problem is the inevitable low and non-reproducible assay (purity of -90% on dry basis) of Compound 9-HCl due to the residues of the other two phases. It should be noted that a high assay is important in the next step in order to control the amount of reagents. The third problem is the existence of THF in the wet Compound 9-HCl salt which is responsible for the Compound 3 impurity that is discussed below.
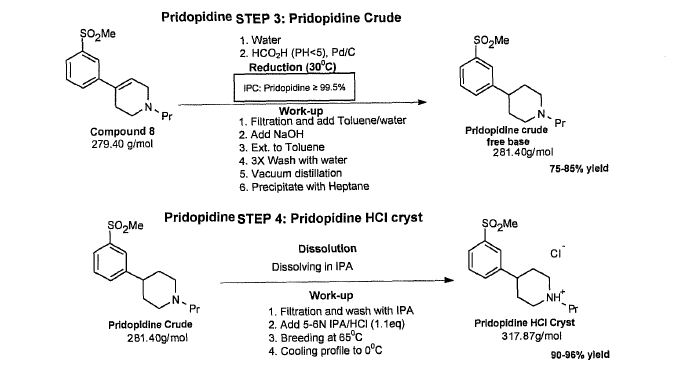
Example 6.2: Pridopidine crude – work-up development
After the reduction, pridopidine HC1 is precipitated by adding HC1/IPA to the solution of pridopidine free base in ΓΡΑ in the process of Example 1. Prior to that, a solvent swap from toluene to ΓΡΑ is completed by 3 consecutive vacuum distillations. The amount of toluene in the ΓΡΑ solution affects the yield and it was set to be not more than 3% (IPC by GC method). The spontaneous precipitation produces fine crystals with wide PSD. In order to narrow the PSD, Example 1 accomplishes HC1/IPA addition in two cycles with cooling/warming profile.
The updated process is advantageous for crystallizing pridopidine free base over the procedure in Example 1 for two reasons.
First, it simplifies the work-up of the crude because the swap from toluene to PA is not required. The pridopidine free base is crystallized from toluene/n-heptanes system. Only one vacuum distillation of toluene is needed (compared to three in the work-up of Example 1) to remove water and to increase yield.
Second, in order to control pridopidine-HCl physical properties. Pridopidine free base is a much better starting material for the final crystallization step compared to the pridopidine HC1 salt because it is easily dissolved in ΓΡΑ which enables a mild absolute (0.2μ) filtration required in the final step of API manufacturing.
Crystallization of pridopidine free base in toluene/n-heptane system
First, crystallization of pridopidine free base in toluene/n-heptane mixture was tested in order to find the right ratio to maximize the yield. In order to obtain pridopidine free base, pridopidine-HCl in water/toluene system was basified with NaOH(aq) to pH>12. Two more water washes of the toluene phase brought the pH of the aqueous phase to <10. Addition of n-heptane into the toluene solution
resulted in pridopidine free base precipitation. Table 21 shows data from the toluene/n-heptane crystallization experiments.
Example 7: Development of the procedure for the purification of Compound 1 in pridopidine free base.
The present example describes lowering Compound 1 levels in pridopidine free base. This procedure involves dissolving pridopidine FB in 5 Vol of toluene at 20-30°C, 5 Vol of water are added and after the mixing phases are separated and the organic phase is washed three times with 5 Vol water. The toluene mixture is then distilled up to 2.5 Vol in the reactor and 4 Vol of heptane are added for crystallization. Experiment No. 2501 was completed using this procedure. Table 24 summarizes the results.
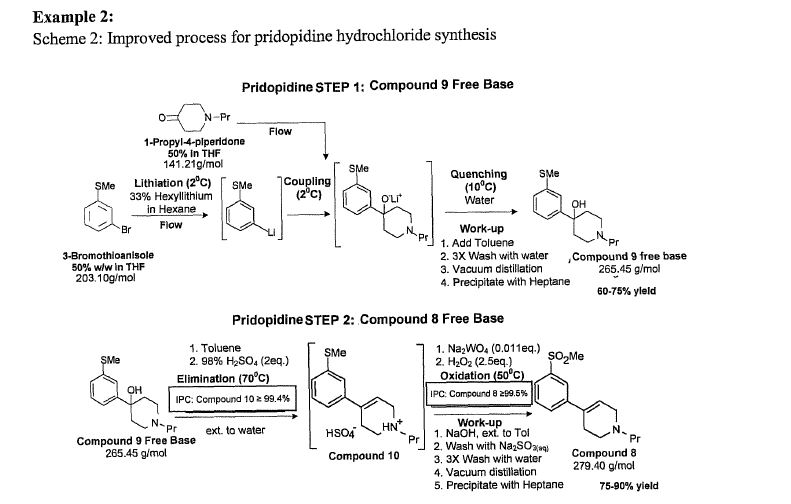
Example 8: Step 4 in Scheme 2: Pridopidine Hydrochloride process
This example discusses the step used to formulate pridopidine-HCl from pridopidine crude. The corresponding stage in Example 1 was part of the last (third) stage in which pridopidine-HCl was obtained directly from Compound 8 without isolation of pridopidine crude. In order to better control pridopidine-HCl physical properties, it is preferable to start with well-defined pridopidine free base which enables control on the exact amount of HC1 and IPA.
Pridopidine-HCl preparation – present procedure
Pridopidine-HCl was prepared according to the following procedure: Solid pridopidine crude was charged into the first reactor followed by 8 Vol of IPA (not more than (NMT) 0.8% water by KF) and the mixture is heated to Tr =40-45°C (dissolution at Tr = 25-28°C). The mixture was then filtered through a 0.2 μιη filter and transferred into the second (crystallizing) reactor. The first hot reactor was washed with 3.8 Vol of IPA. The wash was transferred through the filter to the second reactor. The temperature was raised to 65-67°C and 1.1 eq of IPA/HCl are added to the mixture (1.1 eq of HC1, from IPA/HCl 5N solution, 0.78 v/w). The addition of EPA HCl into the free base is exothermic; therefore, it was performed slowly, and the temperature maintained at Tr = 60-67°C. After the addition, the mixture was stirred for 15 min and pH is measured (pH<4). If pH adjustment is needed,
0.2 eq of HCl (from IPA/HC1 5 N solution) is optional. At the end of the addition, the mixture was stirred for 1 hour at Tr = 66°C to start sedimentation. If sedimentation does not start, seeding with 0.07% pridopidine hydrochloride crystals is optional at this temperature. Breeding of the crystals was performed by stirring for 2.5 h at Tr =64-67°C. The addition HCl line was washed with 0.4 Vol of ΓΡΑ to give~13 Vol solution. The mixture was cooled to Tr =0°C The solid is filtered and washed with cooled 4.6 Vol ΓΡΑ at LT 5°C. Drying as performed under vacuum (P< ) at 30-60°C to constant weight: Dried pridopidine-HCl was obtained as a white solid.
Purification of Compound 4 during pridopidine-HCl process
A relationship between high temperature in the reduction reaction and high levels of Compound 4 impurity have been observed. A reduction in 50°C leads to 0.25% of Compound 4. For that reason the process of Example 1 limits the reduction reaction temperature to 30±5°C since this is the final step and Compound 4 level should be not more than 0.15%. The present process has another crystallization stage by which Compound 4 can be purified.
PATENT
https://www.google.ch/patents/US20130150406
Pridopidine, i.e. 4-(3-methanesulfonyl-phenyl)-1-propyl-piperidine, is a drug substance currently in clinical development for the treatment of Huntington’s disease. The hydrochloride salt of 4-(3-methanesulfonyl-phenyl)-1-propyl-piperidine and a method for its synthesis is described in WO 01/46145. In WO 2006/040155 an alternative method for the synthesis of 4-(3-methanesulfonyl-phenyl)-1-propyl-piperidine is described. In WO 2008/127188 N-oxide and/or di-N-oxide derivatives of certain dopamine receptor stabilizers/modulators are reported, including the 4-(3-methanesulfonyl-phenyl)-1-propyl-piperidine-1-oxide.
1H NMR PREDICTIONS
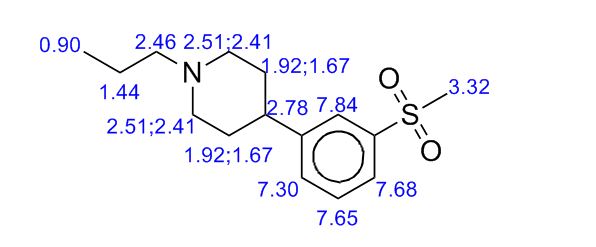
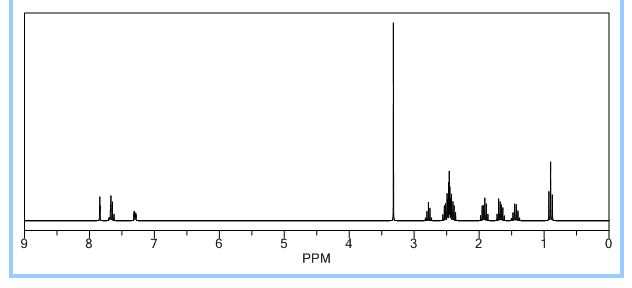
ACTUAL VALUES
13C NMR PREDICTIONS
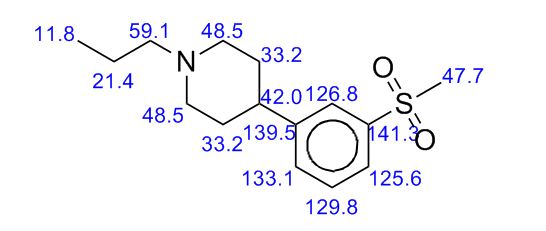
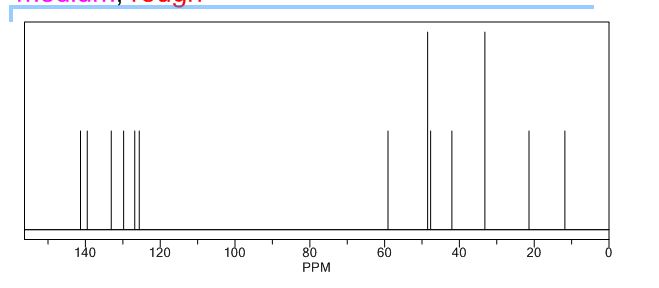
References
- Seeman P, Tokita K, Matsumoto M, Matsuo A, Sasamata M, Miyata K (October 2009). “The dopaminergic stabilizer ASP2314/ACR16 selectively interacts with D2(High) receptors”. Synapse. 63 (10): 930–4. doi:10.1002/syn.20663. PMID 19588469.
- Rung JP, Rung E, Helgeson L, et al. (June 2008). “Effects of (-)-OSU6162 and ACR16 on motor activity in rats, indicating a unique mechanism of dopaminergic stabilization”. Journal of Neural Transmission. 115 (6): 899–908. doi:10.1007/s00702-008-0038-3. PMID 18351286.
- “NeuroSearch A/S announces the results of additional assessment and analysis of data from the Phase III MermaiHD study with Huntexil® in Huntington’s disease” (Press release). NeuroSearch. 28 April 2010. Retrieved 2010-04-28.
- “NeuroSearch press releases (dated 23.03.2011 and 24.05.2011)”. NeuroSearch. “Huntexil update: EMA asks for further trial”. HDBuzz. Retrieved 11 December 2011.
- Ponten, H.; Kullingsjö, J.; Lagerkvist, S.; Martin, P.; Pettersson, F.; Sonesson, C.; Waters, S.; Waters, N. (2003-11-19) [2000-12-22]. “In vivo pharmacology of the dopaminergic stabilizer pridopidine”. European Journal of Pharmacology. 644 (1-3) (1–3): 88–95. doi:10.1016/j.ejphar.2010.07.023. PMID 20667452.
- Dyhring T, Nielsen E, Sonesson C, et al. (February 2010). “The dopaminergic stabilizers pridopidine (ACR16) and (-)-OSU6162 display dopamine D(2) receptor antagonism and fast receptor dissociation properties”. European Journal of Pharmacology. 628 (1–3): 19–26. doi:10.1016/j.ejphar.2009.11.025. PMID 19919834.
- Pettersson, F; Pontén, H; Waters N; Waters S; Sonesson C (March 2010). “Synthesis and Evaluation of a Set of 4-Phenylpiperidines and 4-Phenylpiperazines as D2 Receptor Ligands and the Discovery of the Dopaminergic Stabilizer 4-[3-(methylsulfonyl)phenyl]-1-propylpiperidine (Pridopidine; ACR16)”. Journal of Medicinal Chemistry. 53 (6): 2510–2520. doi:10.1021/jm901689v. PMID 20155917.
- Natesan S, Svensson KA, Reckless GE, et al. (August 2006). “The dopamine stabilizers (S)-(-)-(3-methanesulfonyl-phenyl)-1-propyl-piperidine [(-)-OSU6162] and 4-(3-methanesulfonylphenyl)-1-propyl-piperidine (ACR16) show high in vivo D2 receptor occupancy, antipsychotic-like efficacy, and low potential for motor side effects in the rat”. The Journal of Pharmacology and Experimental Therapeutics. 318 (2): 810–8. doi:10.1124/jpet.106.102905. PMID 16648369.
- Tadori Y, Forbes RA, McQuade RD, Kikuchi T (November 2008). “Characterization of aripiprazole partial agonist activity at human dopamine D3 receptors”. European Journal of Pharmacology. 597 (1–3): 27–33. doi:10.1016/j.ejphar.2008.09.008. PMID 18831971.
- Rung JP, Carlsson A, Markinhuhta KR, Carlsson ML (June 2005). “The dopaminergic stabilizers (-)-OSU6162 and ACR16 reverse (+)-MK-801-induced social withdrawal in rats”. Progress in Neuro-psychopharmacology & Biological Psychiatry. 29 (5): 833–9. doi:10.1016/j.pnpbp.2005.03.003. PMID 15913873.
- Nilsson M, Carlsson A, Markinhuhta KR, et al. (July 2004). “The dopaminergic stabiliser ACR16 counteracts the behavioural primitivization induced by the NMDA receptor antagonist MK-801 in mice: implications for cognition”. Progress in Neuro-psychopharmacology & Biological Psychiatry. 28 (4): 677–85. doi:10.1016/j.pnpbp.2004.05.004. PMID 15276693.
- Pettersson F, Waters N, Waters ES, Carlsson A, Sonesson C (November 7, 2002). The development of a new class of dopamine stabilizers. Society for Neuroscience Annual Conference. Orlando, FL.
- Tedroff, J.; Krogh, P. Lindskov; Buusman, A.; Rembratt, Å. (2010). “Poster 20: Pridopidine (ACR16) in Huntington’s Disease: An Update on the MermaiHD and HART Studies”. Neurotherapeutics. 7: 144. doi:10.1016/j.nurt.2009.10.004.
- “NeuroSearch announces results from an open-label safety extension to the Phase III MermaiHD study of Huntexil® in patients with Huntington’s disease” (Press release). NeuroSearch. 15 September 2010. Retrieved 2010-09-15.
- “The HART study with Huntexil® shows significant effect on total motor function in patients with Huntington’s disease although it did not meet the primary endpoint after 12 weeks of treatment” (Press release). NeuroSearch. 14 October 2010. Retrieved 2010-10-14.
REFERENCES CITED:
U.S. Patent No. 6,903,120
U.S. Patent No. 7,923,459
U.S. Publication No. US-2013-0267552-A1
CSED:25948790, http://w .chemspider.com/Chernical-Stmcture.25948790.
CSID:7971505, http://ww.chemspider.com/Chermcal-Stmcture.7971505.html
Ebenezer et al, Tetrahedron Letters 55 (2014) 5323-5326.
REFERENCES
1: Squitieri F, de Yebenes JG. Profile of pridopidine and its potential in the treatment of Huntington disease: the evidence to date. Drug Des Devel Ther. 2015 Oct 28;9:5827-33. doi: 10.2147/DDDT.S65738. eCollection 2015. PubMed PMID: 26604684; PubMed Central PMCID: PMC4629959.
2: Rabinovich-Guilatt L, Siegler KE, Schultz A, Halabi A, Rembratt A, Spiegelstein O. The effect of mild and moderate renal impairment on the pharmacokinetics of pridopidine, a new drug for Huntington’s disease. Br J Clin Pharmacol. 2016 Feb;81(2):246-55. doi: 10.1111/bcp.12792. Epub 2015 Nov 25. PubMed PMID: 26407011.
3: Shannon KM, Fraint A. Therapeutic advances in Huntington’s Disease. Mov Disord. 2015 Sep 15;30(11):1539-46. doi: 10.1002/mds.26331. Epub 2015 Jul 30. Review. PubMed PMID: 26226924.
4: Sahlholm K, Sijbesma JW, Maas B, Kwizera C, Marcellino D, Ramakrishnan NK, Dierckx RA, Elsinga PH, van Waarde A. Pridopidine selectively occupies sigma-1 rather than dopamine D2 receptors at behaviorally active doses. Psychopharmacology (Berl). 2015 Sep;232(18):3443-53. doi: 10.1007/s00213-015-3997-8. Epub 2015 Jul 11. PubMed PMID: 26159455; PubMed Central PMCID: PMC4537502.
5: Squitieri F, Di Pardo A, Favellato M, Amico E, Maglione V, Frati L. Pridopidine, a dopamine stabilizer, improves motor performance and shows neuroprotective effects in Huntington disease R6/2 mouse model. J Cell Mol Med. 2015 Nov;19(11):2540-8. doi: 10.1111/jcmm.12604. Epub 2015 Jun 22. PubMed PMID: 26094900; PubMed Central PMCID: PMC4627560.
6: Waters S, Ponten H, Klamer D, Waters N. Co-administration of the Dopaminergic Stabilizer Pridopidine and Tetrabenazine in Rats. J Huntingtons Dis. 2014;3(3):285-98. doi: 10.3233/JHD-140108. PubMed PMID: 25300332.
7: Waters S, Ponten H, Edling M, Svanberg B, Klamer D, Waters N. The dopaminergic stabilizers pridopidine and ordopidine enhance cortico-striatal Arc gene expression. J Neural Transm (Vienna). 2014 Nov;121(11):1337-47. doi: 10.1007/s00702-014-1231-1. Epub 2014 May 11. PubMed PMID: 24817271.
8: Reilmann R. The pridopidine paradox in Huntington’s disease. Mov Disord. 2013 Sep;28(10):1321-4. doi: 10.1002/mds.25559. Epub 2013 Jul 11. PubMed PMID: 23847099.
9: Gronier B, Waters S, Ponten H. The dopaminergic stabilizer pridopidine increases neuronal activity of pyramidal neurons in the prefrontal cortex. J Neural Transm (Vienna). 2013 Sep;120(9):1281-94. doi: 10.1007/s00702-013-1002-4. Epub 2013 Mar 7. PubMed PMID: 23468085.
10: Huntington Study Group HART Investigators. A randomized, double-blind, placebo-controlled trial of pridopidine in Huntington’s disease. Mov Disord. 2013 Sep;28(10):1407-15. doi: 10.1002/mds.25362. Epub 2013 Feb 28. PubMed PMID: 23450660.
11: Squitieri F, Landwehrmeyer B, Reilmann R, Rosser A, de Yebenes JG, Prang A, Ivkovic J, Bright J, Rembratt A. One-year safety and tolerability profile of pridopidine in patients with Huntington disease. Neurology. 2013 Mar 19;80(12):1086-94. doi: 10.1212/WNL.0b013e3182886965. Epub 2013 Feb 27. PubMed PMID: 23446684.
12: Ponten H, Kullingsjö J, Sonesson C, Waters S, Waters N, Tedroff J. The dopaminergic stabilizer pridopidine decreases expression of L-DOPA-induced locomotor sensitisation in the rat unilateral 6-OHDA model. Eur J Pharmacol. 2013 Jan 5;698(1-3):278-85. doi: 10.1016/j.ejphar.2012.10.039. Epub 2012 Nov 2. PubMed PMID: 23127496.
13: Lindskov Krog P, Osterberg O, Gundorf Drewes P, Rembratt Å, Schultz A, Timmer W. Pharmacokinetic and tolerability profile of pridopidine in healthy-volunteer poor and extensive CYP2D6 metabolizers, following single and multiple dosing. Eur J Drug Metab Pharmacokinet. 2013 Mar;38(1):43-51. doi: 10.1007/s13318-012-0100-2. Epub 2012 Sep 5. PubMed PMID: 22948856.
14: Ruiz C, Casarejos MJ, Rubio I, Gines S, Puigdellivol M, Alberch J, Mena MA, de Yebenes JG. The dopaminergic stabilizer, (-)-OSU6162, rescues striatal neurons with normal and expanded polyglutamine chains in huntingtin protein from exposure to free radicals and mitochondrial toxins. Brain Res. 2012 Jun 12;1459:100-12. doi: 10.1016/j.brainres.2012.04.021. Epub 2012 Apr 21. PubMed PMID: 22560595.
15: Helldén A, Panagiotidis G, Johansson P, Waters N, Waters S, Tedroff J, Bertilsson L. The dopaminergic stabilizer pridopidine is to a major extent N-depropylated by CYP2D6 in humans. Eur J Clin Pharmacol. 2012 Sep;68(9):1281-6. doi: 10.1007/s00228-012-1248-z. Epub 2012 Mar 8. PubMed PMID: 22399238.
16: Sahlholm K, Århem P, Fuxe K, Marcellino D. The dopamine stabilizers ACR16 and (-)-OSU6162 display nanomolar affinities at the σ-1 receptor. Mol Psychiatry. 2013 Jan;18(1):12-4. doi: 10.1038/mp.2012.3. Epub 2012 Feb 21. PubMed PMID: 22349783.
17: Neurodegenerative disease: Pridopidine for Huntington disease falls short of primary efficacy end point in phase III trial. Nat Rev Neurol. 2011 Dec 26;8(1):4. doi: 10.1038/nrneurol.2011.208. PubMed PMID: 22198402.
18: de Yebenes JG, Landwehrmeyer B, Squitieri F, Reilmann R, Rosser A, Barker RA, Saft C, Magnet MK, Sword A, Rembratt A, Tedroff J; MermaiHD study investigators. Pridopidine for the treatment of motor function in patients with Huntington’s disease (MermaiHD): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011 Dec;10(12):1049-57. doi: 10.1016/S1474-4422(11)70233-2. Epub 2011 Nov 7. PubMed PMID: 22071279.
19: Feigin A. Pridopidine in treatment of Huntington’s disease: beyond chorea? Lancet Neurol. 2011 Dec;10(12):1036-7. doi: 10.1016/S1474-4422(11)70247-2. Epub 2011 Nov 7. PubMed PMID: 22071278.
20: Esmaeilzadeh M, Kullingsjö J, Ullman H, Varrone A, Tedroff J. Regional cerebral glucose metabolism after pridopidine (ACR16) treatment in patients with Huntington disease. Clin Neuropharmacol. 2011 May-Jun;34(3):95-100. doi: 10.1097/WNF.0b013e31821c31d8. PubMed PMID: 21586914.
| US6903120 | Dec 22, 2000 | Jun 7, 2005 | A. Carlsson Research Ab | Modulators of dopamine neurotransmission |
| US7417043 | Dec 21, 2004 | Aug 26, 2008 | Neurosearch Sweden Ab | Modulators of dopamine neurotransmission |
| US7923459 | Apr 10, 2007 | Apr 12, 2011 | Nsab, Filial Af Neurosearch Sweden Ab, Sverige | Process for the synthesis of 4-(3-methanesulfonylphenyl)-1-N-propyl-piperidine |
| US20070238879 * | Apr 10, 2007 | Oct 11, 2007 | Gauthier Donald R | Process for the synthesis of 4-(3-methanesulfonylphenyl)-1-n-propyl-piperidine |
| US20100105736 | Apr 14, 2008 | Apr 29, 2010 | Nsab, Filial Af Neurosearch Sweden Ab, Sverige | N-oxide and/or di-n-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| US20130150406 | Dec 7, 2012 | Jun 13, 2013 | IVAX International GmbH | Hydrobromide salt of pridopidine |
| US20130197031 | Aug 31, 2011 | Aug 1, 2013 | IVAX International GmbH | Deuterated analogs of pridopidine useful as dopaminergic stabilizers |
| US20130267552 | Apr 3, 2013 | Oct 10, 2013 | IVAX International GmbH | Pharmaceutical compositions for combination therapy |
| US20140088140 | Sep 27, 2013 | Mar 27, 2014 | Teva Pharmaceutical Industries, Ltd. | Combination of laquinimod and pridopidine for treating neurodegenerative disorders, in particular huntington’s disease |
| US20140088145 | Sep 27, 2013 | Mar 27, 2014 | Teva Pharmaceutical Industries, Ltd. | Combination of rasagiline and pridopidine for treating neurodegenerative disorders, in particular huntington’s disease |
| CN101056854A | Oct 13, 2005 | Oct 17, 2007 | 神经研究瑞典公司 | Process for the synthesis of 4-(3-methanesulfonylphenyl)-1-N-propyl-piperidine |
| WO2001046145A1 | Dec 22, 2000 | Jun 28, 2001 | A. Carlsson Research Ab | New modulators of dopamine neurotransmission |
| WO2006040155A1 | Oct 13, 2005 | Apr 20, 2006 | Neurosearch Sweden Ab | Process for the synthesis of 4-(3-methanesulfonylphenyl)-1-n-propyl-piperidine |
| US9006445 | 6. Sept. 2012 | 14. Apr. 2015 | IVAX International GmbH | Polymorphic form of pridopidine hydrochloride |
| US9139525 | 11. Apr. 2008 | 22. Sept. 2015 | Teva Pharmaceuticals International Gmbh | N-oxide and/or di-N-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| US20100105736 * | 14. Apr. 2008 | 29. Apr. 2010 | Nsab, Filial Af Neurosearch Sweden Ab, Sverige | N-oxide and/or di-n-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| US20160176821 * | 18. Dez. 2015 | 23. Juni 2016 | Teva Pharmaceuticals International Gmbh | L-tartrate salt of pridopidine |
| USRE46117 | 22. Dez. 2000 | 23. Aug. 2016 | Teva Pharmaceuticals International Gmbh | Modulators of dopamine neurotransmission |
| WO2014205229A1 * | 19. Juni 2014 | 24. Dez. 2014 | IVAX International GmbH | Use of high dose pridopidine for treating huntington’s disease |
| WO2015112601A1 * | 21. Jan. 2015 | 30. Juli 2015 | IVAX International GmbH | Modified release formulations of pridopidine |
| WO2016106142A1 * | 18. Dez. 2015 | 30. Juni 2016 | Teva Pharmaceuticals International Gmbh | L-tartrate salt of pridopidine |
 |
|
| Names | |
|---|---|
| IUPAC name
4-(3-(Methylsulfonyl)phenyl)-1-propylpiperidine
|
|
| Identifiers | |
| 346688-38-8 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7971505 |
| KEGG | D09953 |
| PubChem | 9795739 |
| UNII | HD4TW8S2VK |
| Properties | |
| C15H23NO2S | |
| Molar mass | 281.41 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
/////////pridopidine, PHASE 3, TEVA, 346688-38-8, orphan drug designation, Neurosearch, ACR16, Huntexil, ASP 2314, FR 310826, UNII-HD4TW8S2VK
CCCN1CCC(CC1)c2cccc(c2)S(C)(=O)=O
OXIDE
Example 5 – Preparation Of Compound 5 (4-(3-(methylsulfonyl)phenyl)-l-propylpiperidine 1-oxide)

Pridopidine (50.0g, 178mmol, leq) was dissolved in methanol (250mL) and 33% hydrogen peroxide (20mL, 213mmol, 1.2eq). The reaction mixture was heated and kept at 40°C for 20h. The reaction mixture was then concentrated in a rotavapor to give 71g light-yellow oil. Water (400mL) was added and the suspension was extracted with isopropyl acetate (150mL) which after separation contains unreacted pridopidine while water phase contains 91% area of Compound 5 (HPLC). The product was then washed with dichloromethane (400mL) after adjusting the water phase pH to 9 by sodium hydroxide. After phase separation the water phase was washed again with dichloromethane (200mL) to give 100% area of Compound 5 in the water phase (HPLC). The product was then extracted from the water phase into butanol (lx400mL, 3x200ml) and the butanol phases were combined and concentrated in a rotavapor to give 80g yellow oil (HPLC: 100% area of Compound 5). The oil was washed with water (150mL) to remove salts and the water was extracted with butanol. The organic phases were combined and concentrated in a rotavapor to give 43g of white solid which was suspended in MTBE for lhr, filtered and dried to give 33g solid that was melted when standing on air. After high vacuum drying (2mbar, 60°C, 2.5h) 32.23g pure Compound 5 were obtained (HPLC: 99.5% area, 1H-NMR assay: 97.4%).
NMR Identity Analysis of Compound 5
Compound 5: 
The following data in Tables 10 and 11 was determined using a sample of 63.06 mg Compound 5, a solvent of 1.2 ml DMSO-D6, 99.9 atom%D, and the instrument was a Bruker Avance ΙΠ 400 MHz.
Table 10: Assignment of ¾ NMRa,c

a The assignment is based on the coupling pattern of the signals, coupling constants and chemical shifts.
b Weak signal.
c Spectra is calibrated by the solvent residual peak (2.5 ppm).
Table 11: Assignment of 13C NMRa,b

a The assignment is based on the chemical shifts and 1H-13C couplings extracted from HSQC and HMBC experiments.
b Spectra is calibrated by a solvent peak (39.54 ppm)
PATENT
http://www.google.bg/patents/WO2013086425A1?cl=en&hl=bg
Preparation of pridopidine HBr
In order to prepare 33 g of pridopidine HBr, 28.5 g of free base was dissolved in 150 ml 99% ethanol at room temperature. 1 .5 equivalents of hydrobromic acid 48% were added. Precipitation occurred spontaneously, and the suspension was left in refrigerator for 2.5 hours. Then the crystals were filtered, followed by washing with 99% ethanol and ether. The crystals were dried over night under vacuum at 40°C: m.p. 196°C. The results of a CHN analysis are presented in Table 2, below.
NMR 1 H NMR (DMSO-d6): 0.93 ( 3H, t), 1 .68-1 .80 ( 2H, m), 1 .99-2.10 ( 4H, m) 2.97-3.14 (5H, m), 3.24 ( 3H, s), 3.57-3.65 ( 2H, d), 7.60-7.68 (2H, m), 7.78-7.86 ( 2H, m) and 9.41 ppm (1 H, bs).