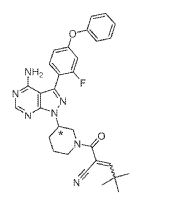
PRN-473
SAR-444727
1414354-91-8
(3R)-3-[4-Amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-α-(2,2-dimethylpropylidene)-β-oxo-1-piperidinepropanenitrile
2-(3-(4-amino~3-(2-fiuoro~4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carbonyl)-4,4-dimethylpent-2-enenitrile
2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile
- OriginatorPrincipia Biopharma
- ClassSmall molecules
- Mechanism of ActionAgammaglobulinaemia tyrosine kinase inhibitors
- Phase IAutoimmune disorders
- DiscontinuedArthritis
- 28 Sep 2020Principia Biopharma has been acquired by Sanofi
- 22 Jun 2020Principia Biopharma plans a pharmacokinetic phase I trial (In volunteers) for Hypersensitivity (for Immunoglobulin E-mediated allergies) in Australia (Topical) (ACTRN12620000693921)
- 10 Mar 2020Phase-I clinical trials in Autoimmune disorders (In volunteers) in Australia (Topical)
- US 8957080
- US 8673925
- WO 2014022569
- WO 2013191965
- WO 2012158764
Useful for treating pemphigus vulgaris, immune thrombocytopenia, inflammatory bowel disease, Sjogren’s syndrome, multiple sclerosis, chronic lymphocytic leukemia and ankylosing spondylitis. Principia Biopharma is developing a topical formulation PRN-473 (presumed to be SAR-444727), a reversible covalent bruton’s (BTK) tyrosine kinase inhibitor, developed based on Principia’s reversible, tailored covalency platform, for treating immune-mediated diseases [phase I, July 2021]. Principia Biopharma was also investigating BTK inhibitors , developed based on Principia’s reversible, tailored covalency platform, for treating hematologic malignancies [no development reported since July 2019]. At the time of publication, Zhu was also affiliated with Nurix Therapeutics , while By and Phiasivongsa were based at Rain Therapeutics and Kronos Bio , respectively.
PATENT
WO-2021142131
Novel crystalline polymorphic forms (I to V) of PRN-473 and their preparation method.
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021142131&_cid=P22-KR7EAJ-62766-1
CRYSTALLINE FORMS OF 2- [3- [4- AMINO-3-(2- FLUORO-4-PHENOXY- PHENYL)-1H-PYRAZOLO[3,4-D]PYRIMIDIN-1-YL]PIPERIDINE-1-CARBONYL]- 4,4-DIMETHYLPENT-2-ENENITRILE
This application claims the benefit of priority to U.S. Provisional Application No. 62/958,389, filed January 8, 2020, the contents of which are incorporated by reference herein in their entirety.
Disclosed herein are crystalline forms of 2-(3-(4-amino~3-(2-fiuoro~4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carbonyl)-4,4-dimethylpent-2-enenitrile (Compound (I)), methods of using the same, and processes for making Compound (I), including its various crystalline forms. The crystalline forms of Compound (I) are inhibitors of Bruton’s tyrosine kinase (BTK). The enzyme BTK is a member of the Tec family non-receptor tyrosine kinases.
BTK is expressed in most hematopoietic cells, including B cells, mast cells, and macrophages. BTK plays a role in the development and activation of B cells and has been implicated in multiple signaling pathways across a wide range of immune-mediated diseases. BTK activity has been implicated in the pathogenesis of several disorders and conditions, such as B cel1-related hematological cancers (e.g,, non-Hodgkin lymphoma and B cell chronic lymphocytic leukemia) and autoimmune diseases (e.g, rheumatoid arthritis,
Sjogren’s syndrome, pemphigus, IBD, lupus, and asthma).
Compound (I) and various solid forms thereof may inhibit BTK and be useful in the treatment of disorders and conditions mediated by BTK activity. Compound (I) is disclosed as, e.g., Compound 125A in Table 1 of WO 2012/158764 and has the following structure:

Example 1: Preparation of Crystalline Form (I) of Compound (I)
Methyl isobutyl ketone (MIBK; 6 mL) was added to amorphous (R)-2-(3-(4-amino-3- (2-fluoro-4-phenoxyphenyJ)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carbonyl)-4,4- dimethylpent-2-enenitrile (1,0 g) and stirred to fonn a solution. After approximately five minutes of agitation, a precipitate began to form. Additional MIBK (10 mL) was charged, and the slurry was stirred. After approximately ten days, the solid was filtered and rinsed with MIBK (10 mL). The solid was dried under vacuum with heating to afford approximately 0.5 g of crystalline Form (I) of Compound (I) as a white solid.
PATENT
WO2012158764 , claiming BTK tyrosine kinase inhibitors, useful for treating cancer.
https://patents.google.com/patent/WO2012158764A1/en
WO 2012/158764 125A

PATENT
US20210205313
PATENT
US20210205312 ,
for concurrently published filings, claiming a gel composition comprising PRN-473 and use of another BTK tyrosine kinase inhibitor ie PRN1008 , respectively.
PATENT
WO2016100914 , claiming use of a BTK inhibitor ie PRN-473, alone or in combination with corticosteroid therapy, for treating pemphigus vulgaris.
PATENT
WO 2014022569
https://patents.google.com/patent/WO2014022569A1/en
//////// PRN-473, PRN 473, SAR 444727, PHASE 1
CC(C)(C)C=C(C#N)C(=O)N1CCC[C@H](C1)n1nc(c2c(N)ncnc21)c1ccc(Oc2ccccc2)cc1F
PRN 1008
Example 31 [WO2014039899] | PRN-1008 | PRN1008
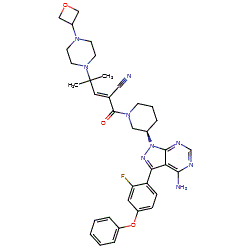
Rilzabrutinib (PRN1008) is an oral, reversible covalent inhibitor of Bruton’s tyrosine kinase (BTK) [1]. The chemical structure of PRN1008 has not been formally disclosed, and there is some uncertainty of its precise stereochemistry evident in online resources. One possible structure is claimed as Example 31 in Principia Biopharma patent WO2014039899 [2] and this is depicted in PubChem CID 118325989 as the R form without E/Z specification. We show the (E,R) structure here.
N#CC(=CC(N(C1COC1)C)(C)C)C(=O)N1CCCC1Cn1nc(c2c1ncnc2N)c1ccc(cc1F)Oc1ccccc1















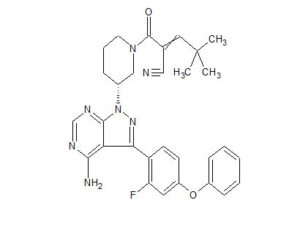
![2-[(3R)-3-[4-Amino-3-(2-fluoro-4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile.png](https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=123342594&t=l)