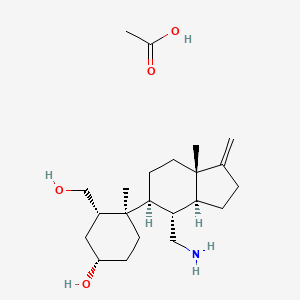
Rosiptor acetate
CAS: 782487-29-0 (acetate) 782487-28-9 (free base)
Chemical Formula: C22H39NO4
Molecular Weight: 381.557
AQX-1125; AQX 1125; AQX1125; AQX-1125 acetate; Rosiptor acetate
PHASE 3 …..a SH2-containing inositol 5-phosphatase 1 (SHIP1) modulator for treating cancer, inflammatory disorders and immune disorders.
(1S,3S,4R)-4-((3aS,4R,5S,7aS)-4-(Aminomethyl)-7a-methyl-1- methyleneoctahydro -1H – inden-5-yl)-3-(hydroxymethyl)-4-methylcyclohexanol, acetate
IUPAC/Chemical Name: (1S,3S,4R)-4-((3aS,4R,5S,7aS)-4-(aminomethyl)-7a-methyl-1-methyleneoctahydro-1H-inden-5-yl)-3-(hydroxymethyl)-4-methylcyclohexan-1-ol acetate
- Originator Aquinox Pharmaceuticals
- Class Anti-inflammatories; Immunotherapies; Small molecules
- Mechanism of Action Inositol-1,4,5-trisphosphate 5-phosphatase stimulants
Highest Development Phases
- Phase III Interstitial cystitis
- Phase II Allergic asthma
- Discontinued Atopic dermatitis; Chronic obstructive pulmonary disease; Haematological disorders; Hypersensitivity; Immunological disorders; Inflammation; Irritable bowel syndrome; Pulmonary fibrosis
Most Recent Events
- 09 Mar 2017 Phase-III clinical trials in Interstitial cystitis in United Kingdom, Poland, Latvia and Canada before March 2017 (PO) (EudraCT2016-000906-12) (NCT02858453)
- 04 Jan 2017 Aquinox Pharmaceuticals completes a phase I trial in Healthy volunteers in United Kingdom (NCT03185195)
- 07 Sep 2016 Phase-III clinical trials in Interstitial cystitis in Czech Republic, Hungary, Denmark (PO) (EudraCT2016-000906-12)
Rosiptor, also known as AQX-1125 is a potent and selective SHIP1 activator currently in clinical development.
AQX-1125 inhibited Akt phosphorylation in SHIP1-proficient but not in SHIP1-deficient cells, reduced cytokine production in splenocytes, inhibited the activation of mast cells and inhibited human leukocyte chemotaxis.
AQX-1125 suppresses leukocyte accumulation and inflammatory mediator release in rodent models of pulmonary inflammation and allergy. As shown in the mouse model of LPS-induced lung inflammation, the efficacy of the compound is dependent on the presence of SHIP1. Pharmacological SHIP1 activation may have clinical potential for the treatment of pulmonary inflammatory diseases.
|
Dysregulated activation of the PI3K pathway contributes to inflammatory/immune disorders and cancer. Efforts have been made to develop modulators of PI3K as well as downstream kinases (Workman et al., Nat. Biotechnol. 24, 794-796, 2006; Simon, Cell 125, 647-649, 2006; Hennessy et al., Nat. Rev. Drug. Discov. 4, 988-1004, 2005; Knight et al., Cell 125, 733-747, 2006; Ong et al., Blood (2007), Vol. 110, No. 6, pp 1942-1949). A number of promising new PI3K isoform specific inhibitors with minimal toxicities have recently been developed and used mouse models of inflammatory disease (Camps et al., Nat. Med. 11, 936-943, 2005; Barber et al., Nat. Med. 11, 933-935, 2005) and glioma (Fan et al., Cancer Cell 9, 341-349, 2006). However, because of the dynamic interplay between phosphatases and kinases in regulating biological processes, inositol phosphatase activators represent a complementary, alternative approach to reduce PIP3 levels. Of the phosphoinositol phosphatases that degrade PIP3, SHIP1 is a particularly ideal target for development of therapeutics for treating immune and hemopoietic disorders because of its hematopietic-restricted expression (Hazen et al., Blood 113, 2924-2933, 2009; Rohrschneider et al., Genes Dev. 14, 505-520, 2000).
|

AQX-1125 is useful in treating disorders and conditions that benefit from SHIP1 modulation, such as cancers, inflammatory disorders and conditions and immune disorders and conditions. AQX-1125 is also useful in the preparation of a medicament for the treatment of such disorders and conditions.
Synthetic methods for preparing AQX-1125 are disclosed in U.S. Pat. Nos. 7,601,874 and 7,999,010. There exists, therefore, a need for improved methods of preparing AQX-1125.
| Inventors | Jeffery R Raymond, Kang Han, Yuanlin Zhou, Yuehua He, Bradley Noren, James Gee Ken Yee, |
| Applicant | Inflazyme Pharm Ltd, Jeffery R Raymond, Kang Han, Yuanlin Zhou, Yuehua He, Bradley Noren, James Gee Ken Yee, |
PATENT
Dysregulated activation of the PI3K pathway contributes to
inflammatory/immune disorders and cancer. Efforts have been made to develop modulators of PI3K as well as downstream kinases (Workman et al., Nat. Biotechnol 24, 794-796, 2006; Simon, Cell 125, 647-649, 2006; Hennessy et al., Nat Rev Drug Discov 4, 988-1004, 2005; Knight et al., Cell 125, 733-747, 2006; Ong et al., Blood (2007), Vol. 110, No. 6, pp 1942-1949). A number of promising new PI3K isoform specific inhibitors with minimal toxicities have recently been developed and used in mouse models of inflammatory disease (Camps et al., Nat Med 1 1 , 936-943, 2005; Barber et ai, Nat Med 1 1 , 933-935, 2005) and glioma (Fan et al., Cancer Cell 9, 341-349, 2006). However, because of the dynamic interplay between phosphatases and kinases in regulating biological processes, inositol phosphatase activators represent a complementary, alternative approach to reduce PIP3 levels. Of the phosphoinositol phosphatases that degrade PIP3i SHIP1 is a particularly ideal target for development of therapeutics for treating immune and hemopoietic disorders because of its
hematopietic-restricted expression (Hazen et al., Blood 1 13, 2924-2933, 2009;
Rohrschneider et ai, Genes Dev. 14, 505-520, 2000).
Small molecule SHIP1 modulators have been disclosed, including
sesquiterpene compounds such as pelorol. Pelorol is a natural product isolated from the tropical marine sponge Dactylospongia elegans (Kwak et al., J Nat Prod 63, 1 153-1 156, 2000; Goclik et al., J Nat Prod 63, 1150-1152, 2000). Other reported SHIP1 modulators include the compounds set forth in PCT Published Patent Applications Nos. WO 2003/033517, WO 2004/035601 , WO 2004/092100, WO 2007/147251 , WO 2007/147252, WO 2011/069118, WO 2014/143561 and WO 2014/158654 and in U.S. Patent Nos. 7,601 ,874 and 7,999,010.
While significant strides have been made in this field, there remains a need for effective small molecule SHIP1 modulators.
One such molecule is the acetate salt of (1 S,3S,4 )-4-((3aS,4 ,5S,7aS)-4-(aminomethyl)-7a-methyl-1-methyleneoctahydro-1 /-/-inden-5-yl)-3-(hydroxymethyl)-4-methylcyclohexanol (referred to herein as Compound 1). Compound 1 is a compound with anti-inflammatory activity and is described in U.S. Patent Nos. 7,601 ,874 and 7,999,010, the relevant disclosures of which are incorporated in full by reference in their entirety, particularly with respect to the preparation of Compound 1 ,
pharmaceutical compositions comprising Compound 1 and methods of using
Compound 1.
Compound 1 has the molecular formula, C2oH36N02+ · C2H302“, a molecular weight of 381.5 g/mole
The application is directed to crystalline forms of the acetate salt of (1S,3S,4R)-4-(3aS,4R,5S,7aS)-4-(aminomethyl)-7a- methyl-1-methyleneoctahydro-1H-inden-5-yl)-3-(hydroxymethyl) -4-methylcyclohexanol and processes for their preparation. The compound acts as a SHIP1 modulator and is thus useful in the treatment of cancer or inflammatory and immune disorders and conditions.
PATENT
https://encrypted.google.com/patents/WO1998002450A2?cl=en
SYNTHESIS
WO 199802450
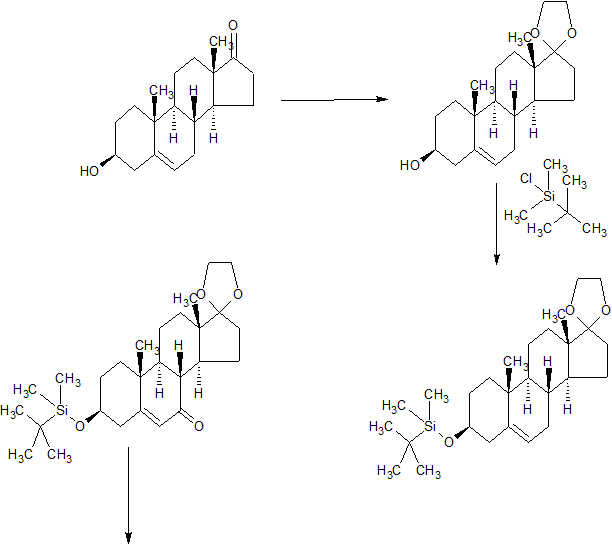
WO 2004092100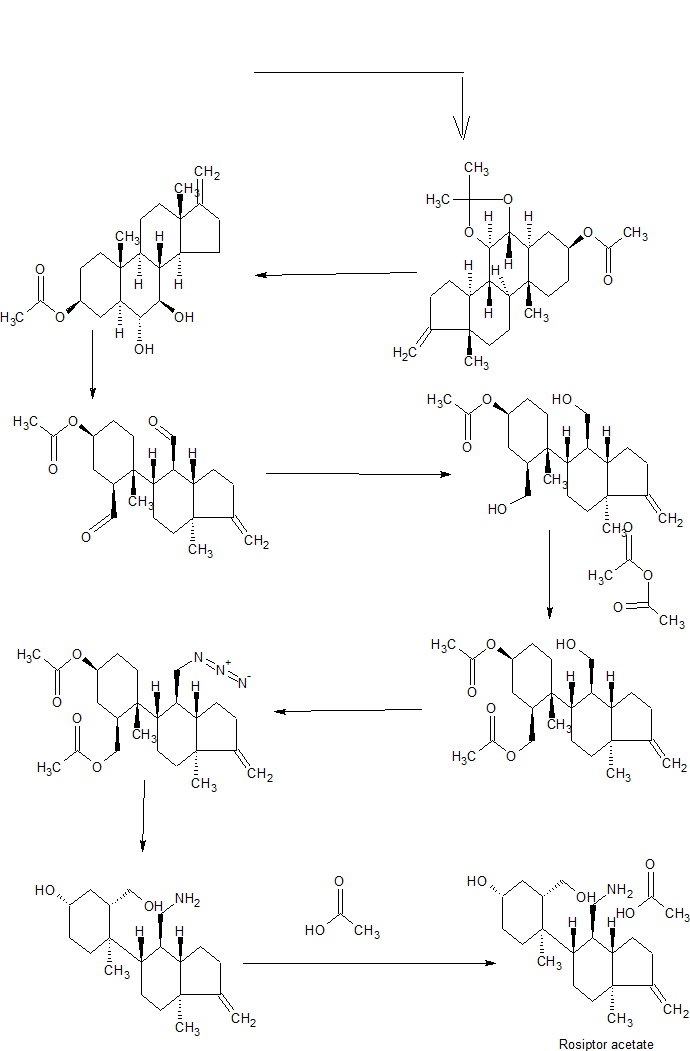
PATENT
WO 2004092100
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2004092100
PATENT
US 20170204048
Process for the synthesis of substituted indene derivative (particularly AQX-1125 ) as a SH2-containing inositol 5-phosphatase 1 (SHIP1) modulator for treating cancer, inflammatory disorders and immune disorders. Aquinox Pharmaceuticals is developing AQX-1125 (phase III clinical trial in July 2017), a SHIP1 agonist, for the treatment of inflammatory diseases. For a prior filing see WO2016210146 , claiming novel crystalline forms of rosiptor acetate. In July 2017, Seenisamy and Chetia were associated with Syngene
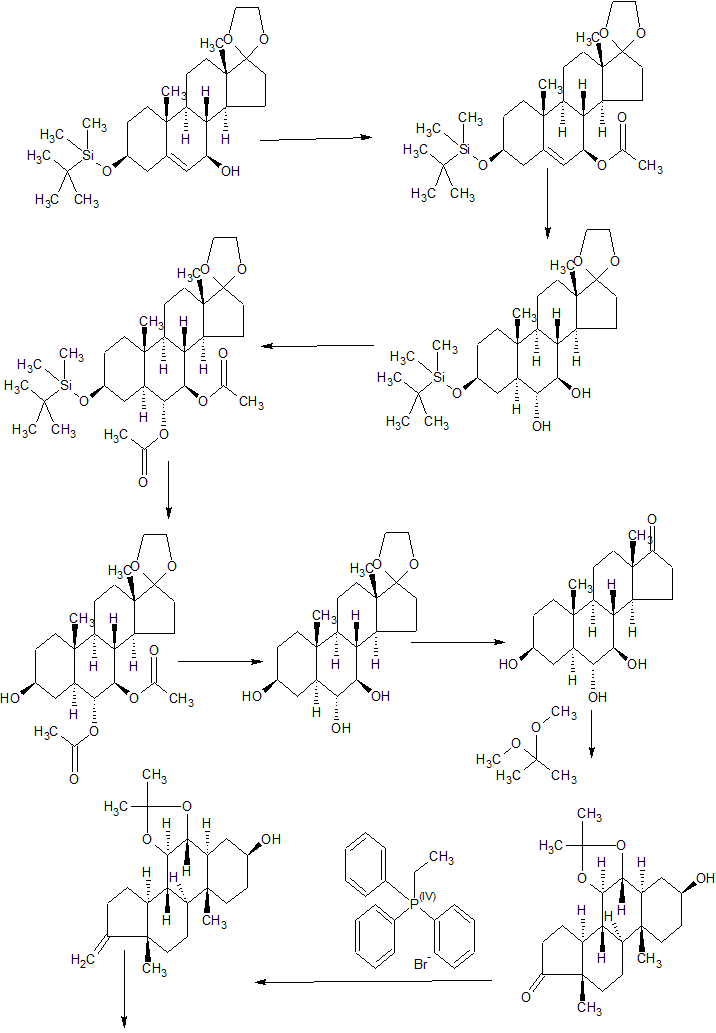
Synthetic Method 1
In one aspect of the invention, AQX-1125 was prepared by the method described below in Reaction Scheme 1 where Pg1 is an oxygen-protecting group, Pg2 is a carbonyl protecting group, Lg1 is a leaving group and X is bromo or chloro:



|
Reaction Scheme 1A:
|



Synthetic Example 77
Step 11: Preparation of AQX-1125 from Compound 16
A. To a stirred solution of (1S,3S,4R)-4-((3aS,4R,5S,7aS)-4-(aminomethyl)-7a-methyl-1-methyleneoctahydro-1H-inden-5-yl)-3-(hydroxymethyl)-4-methylcyclohexan-1-ol (Compound 16, 58.0 g, 0.180 mol, 1.0 eq, from Synthetic Example 76) in methanol (174 mL, 3 V) was added acetic acid (23.5 mL, 0.4 V) dropwise at 10° C. under a nitrogen atmosphere over 20 min. The reaction mixture was stirred at room temperature for 1 h. The solution was filtered to remove undissolved particles and washed with methanol (58 mL, 1 V). The filtrate was collected and evaporated at 35° C. to half the volume (˜125 mL). MTBE (348 mL, 6 V) was slowly added to the above concentrated mixture and the reaction stirred at 10° C. for 2 h. During the MTBE addition, slow precipitation of the product was observed. The solids were filtered and washed with MTBE (116 mL, 2V) to afford (1S,3S,4R)-4-((3aS,4R,5S,7aS)-4-(aminomethyl)-7a-methyl-1-methyleneoctahydro-1H-inden-5-yl)-3-(hydroxymethyl)-4-methylcyclohexan-1-ol, acetic acid salt, (AQX-1125) as a white solid (50 g, yield 72.6%). 1H NMR (400 MHz, pyridine-d5): δ 5.85 (br s, 5H), 4.70 (s, 2H), 4.08 (dd, J=10.4, 2 Hz, 1H), 3.95-3.85 (m, 1H), 3.60-3.50 (m, 1H), 3.18 (d, J=14 Hz, 1H), 2.92-2.86 (m, 1H), 2.80 (d, J=13.6 Hz, 1H), 2.50-2.40 (m, 1H), 2.25-1.97 (m, 3H), 2.15 (s, 3H), 1.90-1.65 (m, 4H), 1.56-1.40 (m, 4H), 1.39-1.20 (m, 2H), 1.25 (s, 3H), 0.78 (s, 3H). LCMS: (Method A) 322.4 (M+1), Retention time: 1.95 min, HPLC (Method H): 95.5 area %, Retention time: 16.66 min.
Synthetic Example 66
Preparation of Compound 16 and AQX-1125
REFERENCES
1: Nickel JC, Egerdie B, Davis E, Evans R, Mackenzie L, Shrewsbury SB. A Phase II Study of the Efficacy and Safety of the Novel Oral SHIP1 Activator AQX-1125 in Subjects with Moderate to Severe Interstitial Cystitis/Bladder Pain Syndrome. J Urol. 2016 Sep;196(3):747-54. doi: 10.1016/j.juro.2016.03.003. PubMed PMID: 26968644.
2: Chuang YC, Chermansky C, Kashyap M, Tyagi P. Investigational drugs for bladder pain syndrome (BPS) / interstitial cystitis (IC). Expert Opin Investig Drugs. 2016;25(5):521-9. doi: 10.1517/13543784.2016.1162290. PubMed PMID: 26940379.
3: Leaker BR, Barnes PJ, O’Connor BJ, Ali FY, Tam P, Neville J, Mackenzie LF, MacRury T. The effects of the novel SHIP1 activator AQX-1125 on allergen-induced responses in mild-to-moderate asthma. Clin Exp Allergy. 2014 Sep;44(9):1146-53. doi: 10.1111/cea.12370. PubMed PMID: 25040039.
4: Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, Toews J, Wu J, Ogden N, MacRury T, Szabo C. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 1. Effects on inflammatory cell activation and chemotaxis in vitro and pharmacokinetic characterization in vivo. Br J Pharmacol. 2013 Mar;168(6):1506-18. doi: 10.1111/bph.12039. PubMed PMID: 23121445; PubMed Central PMCID: PMC3596654.
5: Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, Toews J, Chernoff D, MacRury T, Szabo C. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 2. Efficacy studies in allergic and pulmonary inflammation models in vivo. Br J Pharmacol. 2013 Mar;168(6):1519-29. doi: 10.1111/bph.12038. PubMed PMID: 23121409; PubMed Central PMCID: PMC3596655.
6: Croydon L. BioPartnering North America–Spotlight on Canada. IDrugs. 2010 Mar;13(3):159-61. PubMed PMID: 20191430.
AQX-1125
The SHIP1 Pathway – Highlighting the Role of AQX-1125
AQX-1125 is our lead product candidate and has generated positive clinical data from three completed clinical trials, including two proof-of-concept trials, one in COPD and one in allergic asthma, demonstrating a favorable safety profile and anti-inflammatory activity. Overall, more than 100 subjects have received AQX-1125. Importantly, our clinical trial results were consistent with the drug-like properties and anti-inflammatory activities demonstrated in our preclinical studies. AQX-1125 is a once daily oral capsule with many desirable drug-like properties. We are currently investigating AQX-1125 in two Phase 2 clinical trials, one in COPD and one in BPS/IC.
Based on our three completed clinical trials, we have demonstrated that AQX-1125:
- has desirable pharmacokinetic, absorption and excretion properties that make it suitable for once daily oral administration;
- is generally well tolerated, exhibiting mild to moderate adverse events primarily related to gastrointestinal upset that resolve without treatment or long-term effects and are reduced by taking the drug candidate with food; and
- has anti-inflammatory properties consistent with those exhibited in preclinical studies and exhibited activity in two trials using two distinct inflammatory challenges.
AQX-1125 is an activator of SHIP1, which controls the PI3K cellular signaling pathway. If the PI3K pathway is overactive, immune cells can produce an abundance of pro-inflammatory signaling molecules and migrate to and concentrate in tissues, resulting in excessive or chronic inflammation. SHIP1 is predominantly expressed in cells derived from bone marrow tissues, which are mainly immune cells. Therefore drugs that activate SHIP1 can reduce the function and migration of immune cells and have an anti-inflammatory effect. By controlling the PI3K pathway, AQX-1125 reduces immune cell function and migration by targeting a mechanism that has evolved in nature to maintain homeostasis of the immune system.
AQX-1125 has demonstrated compelling preclinical activity in a broad range of relevant inflammatory studies including preclinical models of COPD, asthma, pulmonary fibrosis, BPS/IC and inflammatory bowel disease (IBD). In these studies we have seen a meaningful reduction in the relevant immune cells that are the cells that cause inflammation, such as neutrophils, eosinophils and macrophages, and a reduction in the symptoms of inflammation, such as pain and swelling. The activity, efficacy and potency seen with AQX-1125 in most preclinical studies compare favorably to published results with corticosteroids. In addition, AQX-1125 demonstrated compelling activity in the smoke airway inflammation and Bleomycin Fibrosis models, which are known to be steroid refractory, or in other words, do not respond to corticosteroids. We believe this broad anti-inflammatory profile is not typical amongst drugs in development and supports the therapeutic potential for AQX-1125.
In addition to demonstrating strong in vitro and in vivo activity, AQX-1125 was also selected as a lead candidate based on its many desirable drug-like properties. The drug candidate is highly water soluble and does not require complex formulation for oral administration. AQX-1125 has low plasma protein binding, is not metabolized and is excreted unmetabolized in both urine and feces. After oral or intravenous dosing, AQX-1125 reaches high concentrations in respiratory, urinary and gastrointestinal tracts, all of which have mucosal surfaces of therapeutic interest. In humans, AQX-1125 has shown pharmacokinetic properties suitable for once-a-day dosing. In addition, the absorption of the drug candidate is equivalent whether taken with or without food.
///////////rosiptor, AQX-1125, AQX 1125, AQX1125; AQX-1125 acetate, Rosiptor acetate, PHASE 3, SH2-containing inositol 5-phosphatase 1, SHIP1, cancer, inflammatory disorders, immune disorders, 782487-29-0, 782487-28-9, Aquinox
CC(=O)O.C[C@@]1(CC[C@@H](C[C@@H]1CO)O)[C@H]2CC[C@]3([C@H]([C@@H]2CN)CCC3=C)C
CC(=O)