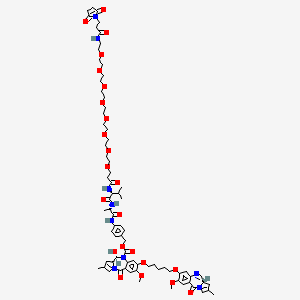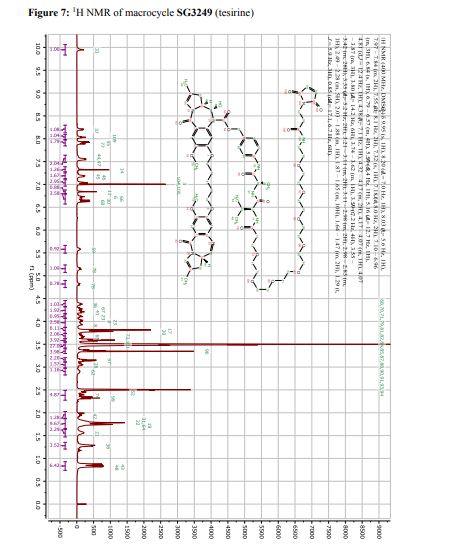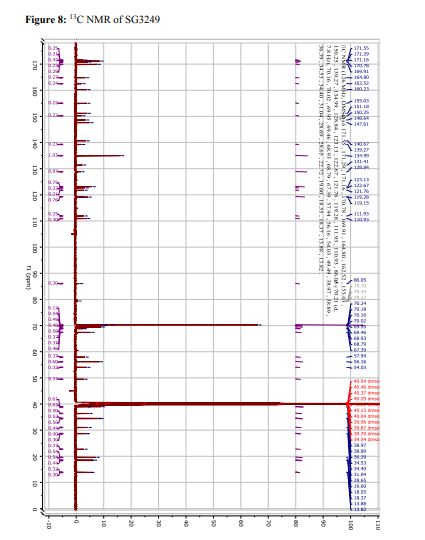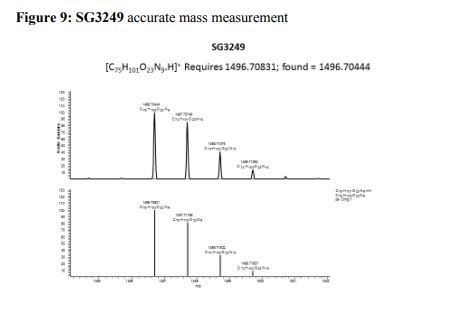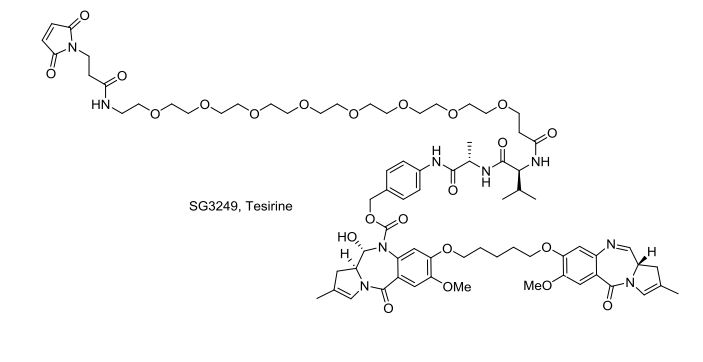
Tesirine
| Molecular Formula: | C75H101N9O23 |
|---|---|
| Molecular Weight: | 1496.673 g/mol |
UNII-8DVQ435K46;
CAS 1595275-62-9
(11S,11aS)-4-((2S,5S)-37-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)-5-isopropyl-2-methyl-4,7,35-trioxo-10,13,16,19,22,25,28,31-octaoxa-3,6,34-triazaheptatriacontanamido)benzyl 11-hydroxy-7-methoxy-8-((5-(((S)-7-methoxy-2-methyl-5-oxo-5,11a-dihydro-1H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-8-yl)oxy)pentyl)oxy)-2-methyl-5-oxo-11,11a-dihydro-1H-benzo[e]pyrrolo[1,2-a][1,4]diazepine-10(5H)-carboxylate
SG3249, Tesirine
[4-[[(2S)-2-[[(2S)-2-[3-[2-[2-[2-[2-[2-[2-[2-[2-[3-(2,5-dioxopyrrol-1-yl)propanoylamino]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]propanoylamino]-3-methylbutanoyl]amino]propanoyl]amino]phenyl]methyl (6S,6aS)-3-[5-[[(6aS)-2-methoxy-8-methyl-11-oxo-6a,7-dihydropyrrolo[2,1-c][1,4]benzodiazepin-3-yl]oxy]pentoxy]-6-hydroxy-2-methoxy-8-methyl-11-oxo-6a,7-dihydro-6H-pyrrolo[2,1-c][1,4]benzodiazepine-5-carboxylate
PATENT
WO 2014057074
In 2012, tesirine (SG3249) was developed by Spirogen, as a drug linker combining a set of desired properties: fast and straightforward conjugation to antibody cysteines by maleimide Michael addition, good solubility in aqueous/DMSO (90/10) systems, and a traceless cleavable linker system delivering the highly potent pyrrolobenzodiazepine (PBD) DNA cross-linker SG3199


CLIP

CLIP
Scale-up Synthesis of Tesirine
This work describes the enabling synthesis of tesirine, a pyrrolobenzodiazepine antibody–drug conjugate drug-linker. Over the course of four synthetic campaigns, the discovery route was developed and scaled up to provide a robust manufacturing process. Early intermediates were produced on a kilogram scale and at high purity, without chromatography. Midstage reactions were optimized to minimize impurity formation. Late stage material was produced and purified using a small number of key high-pressure chromatography steps, ultimately resulting in a 169 g batch after 34 steps. At the time of writing, tesirine is the drug-linker component of eight antibody–drug conjugates in multiple clinical trials, four of them pivotal
.//////////Tesirine, SG3249, SG 3249
CC1=CN2C(C1)C=NC3=CC(=C(C=C3C2=O)OC)OCCCCCOC4=C(C=C5C(=C4)N(C(C6CC(=CN6C5=O)C)O)C(=O)OCC7=CC=C(C=C7)NC(=O)C(C)NC(=O)C(C(C)C)NC(=O)CCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)CCN8C(=O)C=CC8=O)OC