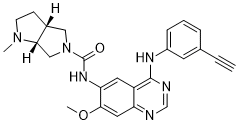
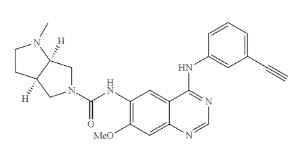
THELIATINIB
CAS: 1353644-70-8
Chemical Formula: C25H26N6O2
Molecular Weight: 442.523
HMPL-309; HMPL 309; HMPL309; Theliatinib.
- Originator Hutchison MediPharma
- Class Antineoplastics; Small molecules
- Mechanism of Action Epidermal growth factor receptor antagonists
Highest Development Phases
- Phase I Oesophageal cancer; Solid tumours
Most Recent Events
- 29 Sep 2017 Efficacy and adverse events data from a phase I trial in Oesophageal cancer released by Hutchison Pharma
- 13 Mar 2017 Phase-I clinical trials in Oesophageal cancer (First-line therapy) in China (PO) before March 2017 (Hutchison MediPharma pipeline, July 2017)
- 02 Aug 2016 Hutchison MediPharma plans a phase Ib proof-of-concept trial for Oesophageal cancer, and Head and Neck cancer in China
Theliatinib, also known as HMPL-309, is a novel small molecule, epidermal growth factor receptor tyrosine kinase inhibitor with potential antineoplastic and anti-angiogenesis activities. In vitro studies suggest that Theliatinib is a potent EGFR kinase inhibitor with good kinase selectivity and in vivo data demonstrated broad spectrum anti-tumor activity via oral dosing in multiple xerographs such as A-431, Bcap-37 and Fadu.
PRODUCT PATENT
- By Zhang, Weihan; Su, Wei-Guo; Yang, Haibin; Cui, Yumin; Ren, Yongxin; Yan, Xiaoqiang
WO2012000356 , covering quinazoline compounds as EGFR inhibitors
https://encrypted.google.com/patents/WO2012000356A1?cl=pt-PT&hl=en&output=html_text
Example 3:
(3aR,6aR)-N-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yl)-l-methyl-hexahydropyrrolo [3,4-b]pyrrole-5(lH)-carboxamide


[060] To a solution of Compound 3-a (40 g, 0.138 mol, prepared according to procedures disclosed in WO2010002845), pyridine (40 mL, 0.495 mol) and DMF (anhydrous, 22 mL) in anhydrous THF (500 mL), was added phenyl carbonochloridate 3-b (22 mL, 0.175 mol) dropwise at -10°C. The mixture was stirred at room temperature for 12 hours. The precipitates were filtered and then suspended in saturated NaHC03 solution (500 mL). The solid was filtered, washed with H20 and EtOAc, and dried in vacuum to give compound 3-c (46 g).
A mixture of compound 3-c (1 g, 2.44 mmol) and compound 3-d (369 mg, 2.92 mmol) in dioxane (30mL) was stirred at 70°C for 5 hours, and then cooled to the ambient temperature. The precipitates were filtered, washed with EtOAc, and dried in vacuum to give compound 3 (0.8 g). MS (m/e): 443.4 (M+l)+.
PATENT
https://patents.google.com/patent/WO2010002845A2/en
PATENT
US 9168253
https://patents.google.com/patent/US9168253
Example 3 (3aR,6aR)—N-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yl)-1-methyl-hexahydropyrrolo[3,4-b]pyrrole-5(1H)-carboxamide
To a solution of Compound 3-a (40 g, 0.138 mol, prepared according to procedures disclosed in WO2010002845), pyridine (40 mL, 0.495 mol) and DMF (anhydrous, 22 mL) in anhydrous THF (500 mL), was added phenyl carbonochloridate 3-b (22 mL, 0.175 mol) dropwise at −10° C. The mixture was stirred at room temperature for 12 hours. The precipitates were filtered and then suspended in saturated NaHCO3solution (500 mL). The solid was filtered, washed with H2O and EtOAc, and dried in vacuum to give compound 3-c (46 g). A mixture of compound 3-c (1 g, 2.44 mmol) and compound 3-d (369 mg, 2.92 mmol) in dioxane (30 mL) was stirred at 70° C. for 5 hours, and then cooled to the ambient temperature. The precipitates were filtered, washed with EtOAc, and dried in vacuum to give compound 3 (0.8 g). MS (m/e): 443.4 (M+1)+.
PATENT
THELIATINIB BY HUTCHISON
The present invention belongs to the field of pharmacy and provides a crystal form of a compound (3aR,6aR)-N-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yl)-1-methyl-hexahydropyrrolo[3,4-b]pyrrole-5(1H)-carboxamide, a pharmaceutical composition thereof, and a preparation method therefor and the use thereof.
(FR)La présente invention concerne le domaine de la pharmacie et fournit une forme cristalline d’un composé (3aR,6aR)-N-(4-(3-éthynylphénylamino)-7-méthoxyquinazolin-6-yl)-1-méthyl-hexahydropyrrolo[3,4-b]pyrrole-5(1H)-carboxamide, une composition pharmaceutique de celui-ci, et son procédé de préparation et son utilisation.
Novel crystalline forms of the compound presumed to be theliatinib , processes for their preparation and compositions comprising them are claimed. Also claimed is their use for treating lung cancer, colon cancer, breast cancer, ovary cancer, prostate cancer, stomach cancer, kidney cancer, liver cancer, brain cancer, esophageal cancer, bone cancer and leukemia.
Hutchison Medipharma is developing theliatinib, a small molecule EGFR tyrosine kinase and AKT cell proliferation pathway inhibitor, for treating cancer, including brain tumor, esophageal tumor and NSCLC; in September 2017, positive preliminary data were presented. Hutchison is also developing epitinib succinate , for treating cancer including glioblastoma.
Patent CN102906086A discloses compound (3aR,6aR)-N-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yl)-1-methyl-hexahydropyrrolo[3 4-b]pyrrole-5(1H)-carboxamide and its preparation method.
Example 3: (3aR, 6aR) -N- (4- (3- ethynyl-phenylamino) -7-methoxy-quinazolin-6-yl) -1-methyl-hexahydro-pyrrolo [3,4-b] pyrrol -5 (IH) – carboxamide
[0102]
[0103] at -10 ° C, to (40g, 0. 138mol, was prepared in accordance with the operation disclosed in W02010002845) Compound 3-a, pyridine (40mL, O. 495mol) and DMF (anhydrous, 22mL) in dry solution (500 mL) in THF dropwise phenyl chloroformate 3-b (22mL, O. 175mol). The mixture was stirred at room temperature for 12h. The precipitate was filtered off, and then it was suspended in saturated NaHCO3 solution (500mL). The solid was filtered off, washed with H2O and EtOAc, and dried in vacuo to give compound 3_c (46g). Compound 3-c (lg, 2. 44mmol) and the compound 3_d (369mg, 2. 92mmol) in a mixture of two anger dioxane (30mL) was stirred at 70 ° C 5 h, then cooled to ambient temperature. The precipitate was filtered off, washed with EtOAc, and dried in vacuo to give compound 3 (O. 8g). MS (m / e): 443. 4 (M + 1) +.

Theliatinib (HMPL-309)
Theliatinib (HMPL-309) is a novel small molecule, epidermal growth factor receptor tyrosine kinase inhibitor with potential antineoplastic and anti-angiogenesis activities. Theliatinib is being developed as an oral formulation for the treatment of solid tumors like non-small cell lung cancer.
Theliatinib pre-clinical studies were conducted in China. In vitro studies suggest that Theliatinib is a potent EGFR kinase inhibitor with good kinase selectivity and in vivo data demonstrated broad spectrum anti-tumor activity via oral dosing in multiple xerographs such as A-431, Bcap-37 and Fadu. Non-clinical safety studies have indicated that Theliatinib is generally well tolerated in animals.
In November 2012, HMP initiated the first-in-human clinical trials of theliatinib.
Patent Citations (4)
REFERENCES
1: Ren Y, Zheng J, Fan S, Wang L, Cheng M, Shi D, Zhang W, Tang R, Yu Y, Jiao L,
Ni J, Yang H, Cai H, Yin F, Chen Y, Zhou F, Zhang W, Qing W, Su W. Anti-tumor
efficacy of theliatinib in esophageal cancer patient-derived xenografts models
with epidermal growth factor receptor (EGFR) overexpression and gene
amplification. Oncotarget. 2017 Apr 19. doi: 10.18632/oncotarget.17243. [Epub
ahead of print] PubMed PMID: 28472779.THELIATINIB, HMPL-309, HMPL 309, HMPL309, Phase I, Oesophageal cancer, Solid tumours
//////
O=C(N1C[C@]2([H])N(C)CC[C@]2([H])C1)NC3=CC4=C(NC5=CC=CC(C#C)=C5)N=CN=C4C=C3OC