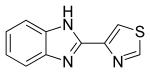
TIABENDAZOLE
CAS: 148-79-8
- Molecular FormulaC10H7N3S
- Average mass201.248 Da
-
тиабендазол [Russian] [INN]تياباندازول [Arabic] [INN]噻苯达唑 [Chinese] [INN]
- チアベンダゾール;
Tiabendazole (INN, BAN), thiabendazole (AAN, USAN), TBZ (and the trade names Mintezol, Tresaderm, and Arbotect) is a preservative[1]
2-Substituted benzimidazole first introduced in 1962. It is active against a variety of nematodes and is the drug of choice for strongyloidiasis. It has CNS side effects and hepatototoxic potential. (From Smith and Reynard, Textbook of Pharmacology, 1992, p919)

Thiabendazole, 2-(4′-thiazolyl)-benzimidazole (TBZ) (I) is an important anthelmintic and fungicidal agent widely used in pharmaceutical, agriculture and food industry. Owing to the commercial importance of thiabendazole, the various synthetic routes are disclosed in the literature for preparing this pharmacologically and fungicidally active compound.
The various literature discloses the synthesis of thiabendazole by using aniline, 4-cyanothiazole and hydrogen chloride in polychlorobenzene such as dichloro- or a trichlorobenzene solvent under high pressure reaction conditions to obtain N-phenyl-(thiazole-4-amidine)-hydrochloride (amidine hydrochloride). This amidine hydrochloride is then treated with hypohalites such as sodium or potassium hypochlorite, sodium hypobromite and calcium hypochlorite in presence of base such as alkali or alkaline earth metal hydroxides such as sodium hydroxide, potassium hydroxide, calcium hydroxide; or an alkali metal carbonate or bicarbonate such sodium carbonate, sodium bicarbonate to obtain thiabendazole.
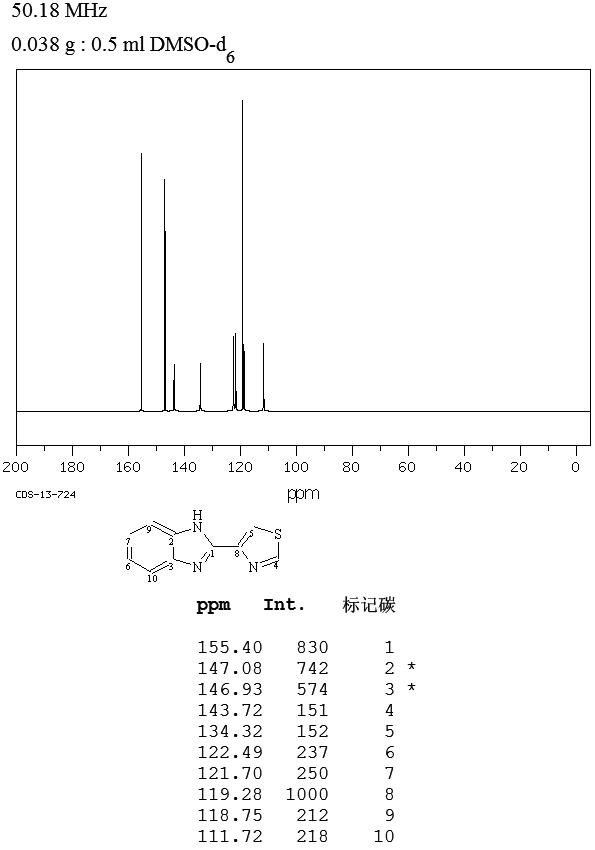
NMR
The US patent no. US 3,274,208 discloses the process for preparation of amidine hydrochloride by reacting 4-cynothiazole and aniline in presence of aluminum chloride at 180 °C. The amidine hydrochloride is purified by acid base treatment.
The US patent no. US 3,299,081 (henceforth patent ‘081) discloses the process for preparation of N-phenyl-(thiazole-4-amidine)-hydrochloride (amidine hydrochloride) and thiabendazole by heating together 4-cyanothiazole and aniline hydrochloride and purging of excess dry hydrogen chloride gas under pressure (15 psig) reaction condition in a 1,2-dichlorobenzene solvent at 135 to 140 °C using closed reactor. The amidine hydrochloride is isolated by filtration and it is then cyclized to N-chloro-N’-phenyl-(thiazole-4-amidine) intermediate by reaction with sodium hypochlorite in water-methanol solvent, further the intermediate is then converted to thiabendazole by treatment with potassium hydroxide in ethanol. The preferred embodiment of the said patent discloses the use of excess hydrogen chloride in a polychlorobenzene medium to achieve higher yields of amidine hydrochloride. The reaction with gas under pressure is exothermic, so the reaction is unsafe.
As per the background of the patent ‘081, the prior art processes were disclosed that the N-aryl amidines could be prepared by reacting together a nitrile and an aromatic amine in the presence of a metal catalyst such as aluminum chloride or zinc chloride. The process involved the use of a metallic halide as an additional substance in the reaction mixture with the result that metal complexes are obtained which have to be decomposed and the metal removed before pure amidine compounds can be recovered. It was also known to prepare N-aryl amidines by reacting the nitrile and the aromatic amine hydrochloride in a solvent such as ether in the absence of metallic halide. The process referred to affords only poor yields of the desired amidine. Hence, neither of these methods are entirely satisfactory.

13C NMR
The US patent no. US 3,299,082 discloses the process for preparation of N-phenyl-(thiazole-4-amidine)-hydrochloride (amidine hydrochloride) by reacting aniline and 4-cyanothiazole in in the presence of a Friedel Crafts type catalyst such as aluminum chloride at temperature 180 °C. The amidine hydrochloride is reacted with hydroxylamine hydrochloride, in presence of base such as sodium bicarbonate and water as solvent to obtain N-phenyl-(thiazole-4-hydroxyamidine) which is then treated with alkyl or aryl sulfonyl halide such methane sulfonyl chloride in the presence of a base such as pyridine to obtain thiabendazole.
The US patent no. US 3,325,506 discloses the process for preparation of thiabendazole by reacting amidine hydrochloride with hypohalites such as sodium or potassium hypochlorite, sodium hypobromite and calcium hypochlorite in presence of base such as alkali or alkaline earth metal hydroxides such as sodium hydroxide, potassium hydroxide, calcium hydroxide; or an alkali metal carbonate or bicarbonate such sodium carbonate, sodium bicarbonate in water or mixtures of water and organic solvents to obtain thiabendazole.
The significance of by-products from reactions in process development work arises from the need to control or eliminate their formation which might affect product cost, process safety, product purity and environmental health. Very few reactions go to 100% completion in the desired sense. Even when conversion is 100% selectivity is not 100%. Most reactions are accompanied by by-products which arise as a direct consequence of a primary synthetic step including work-up and isolation and as a result of various types of side reactions. By-products from the latter type also include tars, polymeric materials, and coloring matters. The level of some by-products from side reactions depends frequently on the batch size.
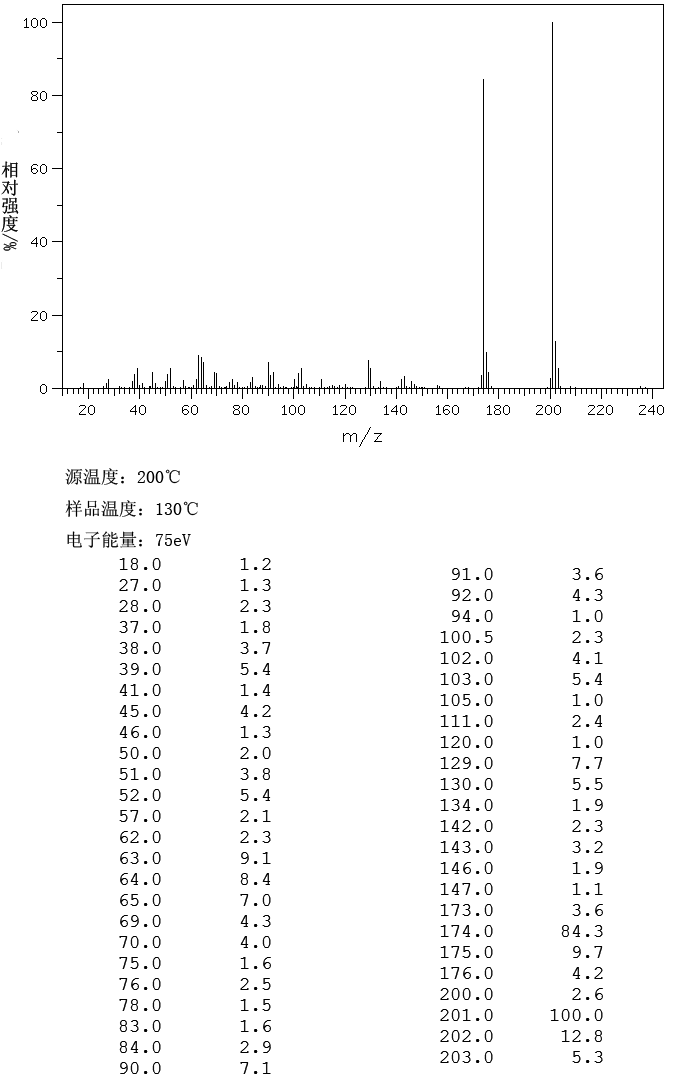
MASS
In the pharmaceutical industry, an impurity is considered as any other inorganic or organic material, or residual solvents other than the drug substances, or ingredients, arise out of synthesis or unwanted chemicals that remains with APIs. Organic impurities are those substances which are formed in the drug substance during the process of synthesis of drug product or even formed during the storage of drug product. This type of impurity includes-intermediate, starting material, degradation product, reagents, ligands, catalyst and by product. Inorganic impurities present mainly include heavy metals, residual solvents, inorganic salts, filter aids, charcoal, reagent, ligands and catalyst.
Impurity profiling includes identification, structure elucidation and quantitative determination of impurities and degradation products in bulk drug materials and pharmaceutical formulations. Impurity profiling has gained importance in modern pharmaceutical analysis since an unidentified, potentially toxic impurities are hazardous to health and the presence of unwanted impurities may influence bioavailability, safety and efficacy of APIs. Now days, not only purity profile but also impurity profile has become mandatory according to various regulatory authorities. The International Conference on Harmonization (ICH) has published guidelines on impurities in new drug substances, products, and residual solvents.
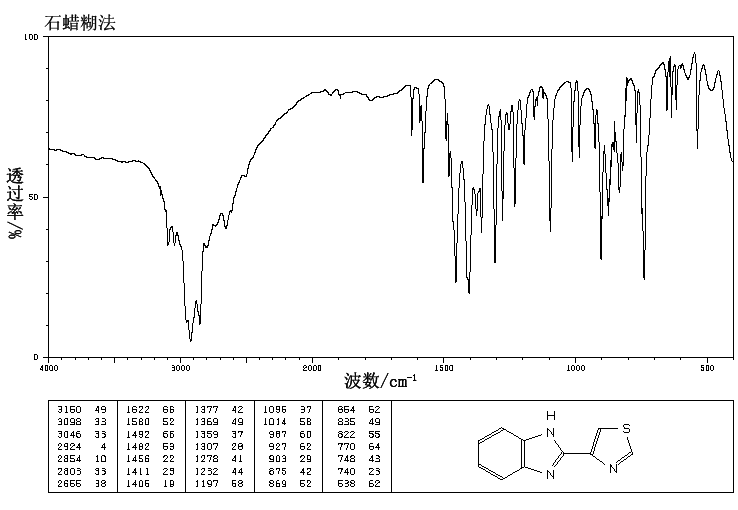
IR
The prior art processes for preparing thiabendazole suffer from inherent drawbacks and inconveniences, such as low yields, additional reaction steps, high-pressure and unsafe reaction conditions. Moreover, the prior art processes for preparation of thiabendazole are end up with surplus level of potential impurities such as 4-chloro thiabendazole (V) or 5-chloro thiabendazole (VI). Also, the prior processes are silent about these impurities. Since, the strict regulations of the regulatory authorities pertaining to the presence of impurities in the active ingredient, it is highly essential to align the research inline with the guidelines of the regulatory authorities in accordance to appropriate regulations and limits to register and commercialize the product in respective countries.
(V) (VI)
Hence, with objective of developing the short process, more direct and less expensive methods, significant improvement in the art for preparation of thiabendazole with controlled level of 4-chloro thiabendazole or 5-chloro thiabendazole impurities, residual solvents (methanol, benzene) and heavy metals (selenium, cobalt, molybdenum), the inventors of the instant invention are motivated to pursue the research to synthesize thiabendazole in under atmospheric conditions with high yield and high chemical purity for agricultural and pharmaceutical use.
CLIP
http://www.inchem.org/documents/jecfa/jecmono/v31je04.htm
Uses
Preservative
It is used primarily to control mold, blight, and other fungal diseases in fruits (e.g. oranges) and vegetables; it is also used as a prophylactic treatment for Dutch elm disease.
Use in treatment of aspergillosis has been reported.[2]
Used in anti-fungal Purple wallboards (optiSHIELD AT, mixture of azoxystrobin and thiabendazole).
Parasiticide
As an antiparasitic, it is able to control roundworms (such as those causing strongyloidiasis),[3] hookworms, and other helminth species which attack wild animals, livestock and humans.[4]
Angiogenesis inhibitor
Genes responsible for the maintenance of cell walls in yeast have been shown to be responsible for angiogenesis in vertebrates. Tiabendazole serves to block angiogenesis in both frog embryos and human cells. It has also been shown to serve as a vascular disrupting agent to reduce newly established blood vessels. Tiabendazole has been shown to effectively do this in certain cancer cells.[5]
Pharmacodynamics
TBZ works by inhibition of the mitochondrial, helminth-specific enzyme, fumarate reductase, with possible interaction with endogenous quinone.[6]
Other
Medicinally, thiabendazole is also a chelating agent, which means it is used medicinally to bind metals in cases of metal poisoning, such as lead, mercury, or antimony poisoning.
In dogs and cats, thiabendazole is used to treat ear infections.
Thiabendazole is also used as a food additive,[7][8] a preservative with E number E233 (INS number 233). For example, it is applied to bananas to ensure freshness, and is a common ingredient in the waxes applied to the skins of citrus fruits. It is not approved as a food additive in the EU,[9] Australia and New Zealand.[10]
Safety
The substance appears to have a slight toxicity in higher doses, with effects such as liver and intestinal disorders at high exposure in test animals (just below LD50 level).[citation needed] Some reproductive disorders and decreasing weaning weight have been observed, also at high exposure. Effects on humans from use as a drug include nausea, vomiting, loss of appetite, diarrhea, dizziness, drowsiness, or headache; very rarely also ringing in the ears, vision changes, stomach pain, yellowing eyes and skin, dark urine, fever, fatigue, increased thirst and change in the amount of urine occur.[citation needed] Carcinogenic effects have been shown at higher doses.[11]
Synthesis
Thiabendazole synthesis:[12] L. H. Sarett, H. D. Brown, U.S. Patent 3,299,081 (1967 to Merck & Co.).
Intermediate arylamidine 2 is prepared by the dry HCl catalyzed addition of aniline to the nitrile function of 4-cyanothiazole (1). Amidine (2) is then converted to its N-chloro analog 3by means of NaOCl. On base treatment, this apparently undergoes a nitrene insertion reaction (4) to produce thiabendazole (5). Note the direction of the arrow is from the benzene to the nitrene since the nitrene is an electrophilic species.
Alternative route of synthesis: 4-thiazolecarboxamide with o-phenylenediamine in polyphosphoric acid.[13]
Synthesis of labeled thiabendazole:[14]
Analogues
Cambendazole preparation and activity studies:[15][16]
Cambendazole (best of 300 agents in an extensive study),[17] is made by nitration of tiabendazole, followed by catalytic hydrogenation to 2, and acylation with Isopropyl chloroformate.
Additionally, tiabendazole was noted to exhibit moderate anti-inflammatory and analgesic activities, which led to the development of KB-1043.
PATENT
WO-2019016834
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019016834&tab=PCTDESCRIPTION&maxRec=1000
The present invention relates to an improved process for preparing thiabendazole of formula (I) with high yield, high purity, in economical and commercially viable manner for agricultural and pharmaceutical use.
Process for preparing thiabendazole with higher yield, purity, in an economical and commercially viable manner. Thiabendazole is an important anthelmintic and fungicidal agent widely used in pharmaceutical, agriculture and food industry. Represents the first filing from the Hikal Ltd and the inventors on thiabendazole.
The structural details of the 4-chloro thiabendazole (V) and 5-chloro thiabendazole (VI) impurities are as follow.
1. 4-Chloro thiabendazole:
(a) FT-IR study: The FT-IR spectrum was recorded in the KBr pellet using ABB FTLA-2000 FT-IR Spectrometer. The IR data is tabulated below.
Frequency (cm“1) Assignment (s)
1576.37 C=C stretching
1309.16 C-N stretching
3073.38 N-H stretching
(b) NMR spectral data:
NMR experiment was carried out on 400 MHz Bruker spectrometer using DMSO as solvent. The chemical shifts are reported on the δ scale in ppm relative DMSO at 2.5 ppm. The 1H spectra displayed in respectively. The NMR assignment of 4-chloro thiabendazole is shown below.
Proton assignments of 4-Chloro thiabendazole:
s-singlet, d-doublet, t -triplet, q- quartet, dd-doublet of doublet, br-broad, m-multiplet.
2. 5-Chloro thiabendazole:
(a) FT-IR study:
The FT-IR spectrum was recorded in the KBr pellet using ABB FTLA- 2000 Spectrometer. The IR data is tabulated below.
(b) NMR spectral data:
NMR experiment was carried out on 400 MHz Bruker spectrometer using DMSO-d6 as solvent. The chemical shifts are reported on the δ scale in ppm relative DMSO-d6 at 2.50
ppm. The 1H spectra displayed in respectively. The NMR assignment of 5-chloro thiabendazole is shown below.
Proton assignments 5-Chloro thiabendazole:
s-singlet, d-doublet, q-quartet m-multiplet, br-broad.
Examples
Example 1: Preparation of amidine hydrochloride (IV)
To the 4-neck, 1 lit RBF, fixed with thermo pocket, condenser and hydrogen chloride (HC1) gas inlet, 100 g (0.908 moles, 1.0 eq) of 4-cyanothiazole, 386 (3.86 V) ml of 1,2-dichlorobenzene and 86.02 (0.924 moles, 1.02 eq) g of aniline were charged. The reaction mass was heated to 55 to 60 °C and hydrogen chloride (HC1) gas was purged till exotherm ceased. Then the temperature of the reaction mass was raised to 135 to 140 °C and again dry HC1 gas was purged till 4-cyanothiazole was reduced to less than 0.2 % (w/w) analyzed by HPLC. The reaction mass was cooled to 45 to 50° C and 500 mL of water was charged and the reaction mass was stirred for half an hour. The pH of the reaction mass was adjusted between 3 to 5 using caustic lye. The reaction mass was filtered through hyflo bed, and bed was washed with 50 (0.5 V) mL of water. The organic layer was separated, and the aqueous layer was charged back to the RBF. 20 g of activated charcoal was added in aqueous layer under stirring at 45 to 50 °C. The reaction mass was heated to 55 to 60 °C and maintained under stirring for 1.0 hour. The reaction mass was filtered through the hyflo bed under
vacuum, and bed was washed with 50 mL of hot water and suck dried till no more filtrate collected. 300-400 mL of water was distilled from the aqueous layer at 55 °C under 50 m bar of vacuum. Then the reaction mass was cooled to 0 to 5 °C and maintained under stirring for 1 hour. The obtain amidine hydrochloride was filtered by using Buckner funnel and suck dried till no more filtrate collected from it. The wet cake was dried under vacuum at 55 to 60 °C to get 189 g (86.83% yield, HPLC purity 99.85%) of amidine hydrochloride.
Example 2: Preparation of thiabendazole (I)
The 5 lit RBF was fixed with over head stirrer, thermo pocket, condenser and addition funnel. 185 g (0.772 moles, 1.0 eq.) of amidine hydrochloride and 1536 mL (7.33V) of water were charged. The reaction mass was cooled to 0 to 5 °C. 1233 mL of methanol was added to the mass and the pH of the reaction mass was adjusted between 9 to 10 by using 5N sodium carbonate solution. The reaction mass was warmed to 10 to 15 °C and 415.35 g (12.57 % w/w, 0.91 eq.) sodium hypochlorite was slowly added by maintaining temperature between 10 to 15 °C. The reaction mass was stirred at same temperature for half an hour. Then the reaction mass was heated to 60 to 65 °C and 46.15 g (12.57 % w/w, 0.1 eq) sodium hypochlorite was added. The reaction mass was stirred at 60 to 65 °C for 1.0 hour and the reaction mass was cooled to 30 to 40 °C. The reaction mass was filtered, the bed was washed with 925 mL of water (5.0 V) and suck dried for 10 minutes to get 238 g (152 g on dry basis, 97.82 % yield, HPLC purity 99.77%) of thiabendazole.
Example 3: Purification of thiabendazole (I)
The 5 lit RBF was fixed with over head stirrer, thermo pocket, condenser and addition funnel. 224 g of wet crude thiabendazole (145 g on dry basis) was charged at 25 to 30 °C. 2392 mL (16.5 V) of water was charged and the reaction mass was heated to 75 to 80 °C. The pH of the reaction mass was adjusted between 1 to 2 by adding concentrated hydrochloride. Then 21.75 g (15 %, w/w) activated charcoal was added and the reaction mass was stirred for 1.0 hour at 75 to 80 °C. The reaction mass was filtered through hyflo bed and the bed was washed with 1445 mL (1.0 V) of hot water. The aqueous layer was charged back to clean RBF and cooled to 0 to 5 °C and stirred for 10 hours. The solid was filtered and suck dried under vacuum to get 224 g wet cake of thiabendazole hydrochloride (135 g on dry basis).
1261 niL (10 V w.r.t dry thiabendazole hydrochloride) was charged and then 224 g wet cake of thiabendazole hydrochloride was added. The reaction mass was heated to 70 to 80 °C and maintained under stirring for half an hour to get clear solution. The pH of the reaction mass was adjusted to 7 to 8 by using liquor ammonia. The reaction mass was cooled to 25 to 30 °C and stirred for 1.0 hour. The reaction mass was filtered, and the wet cake was slurry washed twice with 1350 mL (10V x 2 times). Then the bed was washed with 675 mL (5.0 V) water. The solid was dried under vacuum at 60 to 70 °C to afford 119 g (79.33% yield, HPLC purity 99.96%) of pure thiabendazole.
CLIP

Fig. 5 Raman spectrum of solid thiabendazole, and SERS spectra of ethanol – water solutions on a re-used 3 m m thick Au woodpile array. Spurious bands from impurities are marked with asterisks.
CLIP

Fig. 6 (A) Proton NMR spectrum of thiabendazole in DMSO-d 6 solution. (B) Plots of normalized selective relaxation rate enhancements of H1/ H2, H14, and H12. [TBZ] ¼ 2 Â 10 À3 mol L À1 , [DNA] ¼ 1, 2, 5, 10, 20 Â 10 À5 mol L À1 , pH ¼ 7.4, T ¼ 298 K. (C) Equilibrium constant of the TBZ-DNA system. [DNA] ¼ 2 Â 10 À5 mol L À1 , [TBZ] ¼ 2, 2.5, 3, 3.5, 4 Â 10 À3 mol L À1 , pH ¼ 7.4, T ¼ 298 K.
CLIP
Thiabendazole has been prepared by heating thiazole-4-carboxamide and benzene-1,2-diamine in polyphosphoric acid (Scheme 13) (1961JA(83)1764). An alternative synthesis involves 4-carboxythiazole (CA 162 590253 (2015), CA 62 90958 (1964)) or 4-cyanothiazole (CA 130 110264 (1996), CA 121 57510 (1994)) as starting materials. A different approach to the synthesis of thiabendazole has been described starting from N-arylamidines; in the presence of sodium hypochlorite and a base, N-arylamidine hydrochlorides are transformed to benzimidazoles via formation of N-chloroamidine intermediate followed by ring closure in a stepwise or concerted mechanism (1965JOC(30)259).

One Pot Benzimidazole Synthesis.
A recent report (1) from workers at Chonnam National University (Gwangju, Korea) describes a benzimidazole synthesis which:
- produces good product yields (40-98%, for about 30 examples)
- and proceeds in one pot from three readily available components: sodium azide, an aldehyde, and 2-haloanilines
- shows good functional group tolerance(nitro-, ester-, chloro-, and various heterocyclic functionalities on the aldehyde or haloaniline component).

The Benzimidazole Synthesis of Lee and coworkers (1)
Naturally, there are many established ways to synthesize benzimidazoles, which are important substances used in the design of bioactive substances (2). Recent work has sought to address specific drawbacks associated with these methods, which can include harsh reaction conditions and complicated product mixtures.
Further developments have focused on the use of 2-haloacetanilides, 2-haloarylamidines, arylamino oximes, and N-arylbenzimidamides (3). This work notable due to the useful anthelmintic properties. Anthelmintic agents work to kill or repel intestinal worms. A review (3) discusses the synthesis of benzimidazoles, and cites the breakthrough discovery of thiabendazole by researchers at Merck in 1961. Thiabendazole was found to have potent broad spectrum activity against gastrointestinal parasites.

Early thiabendazole synthesis (3)
The initial synthesis of thiabendazole occured via dehydrative cyclization of 1,2 diaminobenzenze in polyphosphoric acid (PPA). The commercialized process involved the conversion of N-arylamidines using hypochlorite (4). Although this process can be performed in ‘one-pot’ fashion it is more typically performed in two steps.
The ‘one-pot’ benzimidazole synthesis described by Lee et. Al. is showcased by its ability to produce thiabendazole in one step, from readily available starting materials (2-haloanilines, thiazole-4-carboxaldehyde) – in 97% yield.
Their work builds on the report of Driver and coworkers (5) that showed that benzimidazoles could be had from 2-azidoanilines in good yield. Indeed, Lee proposes a mechanism that produces an azidoaldimine intermediate, which foregoes the multistep preparation of 2-azidoaniline starting materials.
One proposed mechanistic pathway is shown, with the following steps:
- initial in situ formation of an aldimine, via addition of aniline to an aldehyde;
- Ar-X insertion of the copper catalyst;
- Cu-azide association, with transfer of azide to the aromatic ring;
- loss of nitrogen with concomitant ring formation, and catalyst regeneration
 One mechanistic explanation proposed by Lee and coworkers (1).
One mechanistic explanation proposed by Lee and coworkers (1).
In developing their method, they investigated a number of factors:
- Solvent. DMSO outperformed other polar solvents (NMP, DMF, DMAc). Less polar solvents failed (toluene, diglyme).
- Source of Copper catalyst. The oxidation state of copper was not a factor, as Cu(I) and Cu(II) salts showed similar performance.
- Ligand Evaluation. Ligand selection was not a large factor. Several were tested; ultimately TMEDA was selected.
- Substituents on the aniline / pyridyl component. Base sensitive substituents were tolerated (benzoate ester) and 3-Cl groups were fine. The sensitivity to a broad range of substituents (the usual EWD- and ED-groups) was not rigorously determined
- Nature of the haloaniline. Although both bromo- and iodoaniline examples were given, the predominance of iodoaniline examples suggests it was prefered by the authors for unstated reasons.
- Reactivity of various aldehyde reactants. Aldehydes of varying classes were evaluated. Yields from aromatic substrates bearing ED groups(benzaldehyde, 4-Cl benzaldehyde, 4-methoxybenzaldehyde) produced the highest product yields. Aliphatic aldehydes produced noticeably lower yields, with the curious exception of pivaldehyde. Several heterocyclic aldehydes (2- furyl- and 2-thionylaldehyde were tested and provided good results.
A synopsis of the Lee Procedure follows:
CuCl (0.1 mmol), haloaniline (2.0 mmol), TMEDA (0.1 mmol), NaN3 (4.0 mmol), aldehyde (2.4 mmol) were combined in DMSO mL), The mixture was heated at 120 C for 12 hours. After cooling to room temperature the mixture was poured onto EtOAc (50 mL), washed with brine (25 mL) and water (25 mL). The organic phase was dried over Mg2SO4, and the residue from evaporation was purified by column chromatography (1:1 hexane / EtOAc mobile phase).
Artie McKim.
(1) Kim, Y.; Kumar, M.R.; Park, N.; Heo, Y.; Lee, S. J. Org. Chem. 2011, 76, 9577-9583.
(2) Tumulty, D.; Cao, K.; Homes, C.P. Org Lett. 2001, 3, 83.; Wu, Z. Rea. P.; Wickham, G.; Tetrahedron Lett. 2000, 41, 9871.; Chari, M.A.; Shobha, P.S.D.; Mukkanti, K. J. Heterocycl. Chem.2010, 47, 153.
(3) Townsend, L.B.; Wise, D.S. Parasitology Today 6, 4 (1990) 107-112.
(4) Grenda, V. J.; Jones, R.E; Gal,G.; Sletzinger J. Org Chem. 30 (1965), 259-261.
(5) Shen, M.; Driver, T.G. Org Lett. 2008, 10, 3367.
References
- ^ “E233 : E Number : Preservative”. www.ivyroses.com. Retrieved 2018-08-28.
- ^ Upadhyay MP, West EP, Sharma AP (January 1980). “Keratitis due to Aspergillus flavus successfully treated with thiabendazole”. Br J Ophthalmol. 64 (1): 30–2. doi:10.1136/bjo.64.1.30. PMC 1039343. PMID 6766732.
- ^ Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, Sánchez-Sánchez P, Matogo-Oyana J, Rodríguez-Calabuig D (December 2004). “Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis”. Expert Opin Pharmacother. 5 (12): 2615–9. doi:10.1517/14656566.5.12.2615. PMID 15571478. Archived from the original on 2016-03-06.
- ^ Portugal R, Schaffel R, Almeida L, Spector N, Nucci M (June 2002). “Thiabendazole for the prophylaxis of strongyloidiasis in immunosuppressed patients with hematological diseases: a randomized double-blind placebo-controlled study”. Haematologica. 87 (6): 663–4. PMID 12031927.
- ^ Cha, HJ; Byrom M; Mead PE; Ellington AD; Wallingford JB; et al. (August 2012). “Evolutionarily Repurposed Networks Reveal the Well-Known Antifungal Drug Thiabendazole to Be a Novel Vascular Disrupting Agent”. PLoS Biology. 10 (8): e1001379. doi:10.1371/journal.pbio.1001379. PMC 3423972. PMID 22927795. Retrieved 2012-08-21.
- ^ Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 970
- ^ Rosenblum, C (March 1977). “Non-Drug-Related Residues in Tracer Studies”. Journal of Toxicology and Environmental Health. 2 (4): 803–14. doi:10.1080/15287397709529480. PMID 853540.
- ^ Sax, N.I. Dangerous Properties of Industrial Materials. Vol 1-3 7th ed. New York, NY: Van Nostrand Reinhold, 1989., p. 3251
- ^ UK Food Standards Agency: “Current EU approved additives and their E Numbers”. Retrieved 2011-10-27.
- ^ Australia New Zealand Food Standards Code“Standard 1.2.4 – Labelling of ingredients”. Retrieved 2011-10-27.
- ^ “Reregistration Eligibility Decision THIABENDAZOLE” (PDF). Environmental Protection Agency. Retrieved 8 January 2013.
- ^ Setzinger, Meyer; Painfield, North; Gaines, Water A.; Grenda, Victor J. (1965). “Novel Preparation of Benzimidazoles from N-Arylamidines. New Synthesis of Thiabendazole1”. The Journal of Organic Chemistry. 30: 259–261. doi:10.1021/jo01012a061.
- ^ Brown, H. D.; Matzuk, A. R.; Ilves, I. R.; Peterson, L. H.; Harris, S. A.; Sarett, L. H.; Egerton, J. R.; Yakstis, J. J.; Campbell, W. C.; Cuckler, A. C. (1961). “Antiparasitic Drugs. Iv. 2-(4′-Thiazolyl)-Benzimidazole, A New Anthelmintic”. Journal of the American Chemical Society. 83 (7): 1764–1765. doi:10.1021/ja01468a052.
- ^ Tocco, D. J.; Buhs, R. P.; Brown, H. D.; Matzuk, A. R.; Mertel, H. E.; Harman, R. E.; Trenner, N. R. (1964). “The Metabolic Fate of Thiabendazole in Sheep1”. Journal of Medicinal Chemistry. 7 (4): 399–405. doi:10.1021/jm00334a002.
- ^ Hoff, Fisher, ZA 6800351 (1969 to Merck & Co.), C.A. 72, 90461q (1970).
- ^ Hoff, D. R.; Fisher, M. H.; Bochis, R. J.; Lusi, A.; Waksmunski, F.; Egerton, J. R.; Yakstis, J. J.; Cuckler, A. C.; Campbell, W. C. (1970). “A new broad-spectrum anthelmintic: 2-(4-Thiazolyl)-5-isopropoxycarbonylamino-benzimidazole”. Experientia. 26 (5): 550–551. doi:10.1007/BF01898506.
- ^ Chronicles of Drug Discovery, Book 1, pp 239-256.
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Mintezol, others |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration |
By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Сmax 1–2 hours (oral administration) |
| Metabolism | GI tract |
| Elimination half-life | 8 hours |
| Excretion | Urine (90%) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| ECHA InfoCard | 100.005.206 |
| Chemical and physical data | |
| Formula | C10H7N3S |
| Molar mass | 201.249 g/mol |
| 3D model (JSmol) | |
| Density | 1.103 g/cm3 |
| Melting point | 293 to 305 °C (559 to 581 °F) |
Synthesis Reference
Lynn E. Applegate, Carl A. Renner, “Preparation of high purity thiabendazole.” U.S. Patent US5310923, issued October, 1977.
/////////////////MK 360, MK-360, NSC-525040, NSC-90507, チアベンダゾール, TIABENDAZOLE, тиабендазол , تياباندازول , 噻苯达唑 ,
















