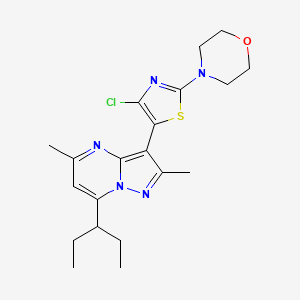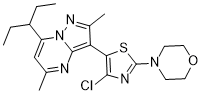
TILDACERFONT
| Synonyms: |
Tildacerfont 1014983-00-6 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-7-(1-ethyl-propyl)-2,5-dimethyl-pyrazolo[1,5-a]pyrimidine 7-(1-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolo[1,5-a]pyrimidine |
|---|---|
| MW/ MF | 420 g/mol/ C20H26ClN5OS |
- Originator Spruce Biosciences
- Class2 ring heterocyclic compounds; Morpholines; Pyrazoles; Pyrimidines; Small molecules; Thiazoles
- Mechanism of Action Corticotropin receptor antagonists
- Orphan Drug Status Yes – Congenital adrenal hyperplasia
- New Molecular Entity Yes
- Phase II Congenital adrenal hyperplasia
- 09 Jul 2020 Spruce Biosciences initiates a phase II trial in Congenital adrenal hyperplasia in USA (PO) (NCT04457336)
- 24 Sep 2019 Spruce Biosciences completes a phase II trial in Congenital adrenal hyperplasia in USA (NCT03687242)
- 19 Sep 2019 Updated safety and efficacy data from a phase II trial in Congenital adrenal hyperplasia release by Spruce Biosciences
Deuterated pyrazolo[1,5-a]pyrimidine derivatives, particularly tildacerfont (SPR-001), useful as CRF antagonists for treating congenital adrenal hyperplasia. Spruce Bioscience is developing tildacerfont under license from Lilly as an oral capsule formulation for the treatment of congenital adrenal hyperplasia; in July 2017, a phase II trial for CAH was initiated.
Corticotropin releasing factor (CRF) is a 41 amino acid peptide that is the primary physiological regulator of proopiomelanocortin (POMC) derived peptide secretion from the anterior pituitary gland. In addition to its endocrine role at the pituitary gland, immunohistochemical localization of CRF has demonstrated that the hormone has a broad extrahypothalamic distribution in the central nervous system and produces a wide spectrum of autonomic, electrophysiological and behavioral effects consistent with a neurotransmitter or neuromodulator role in the brain. There is also evidence that CRF plays a significant role in integrating the response in the immune system to physiological, psychological, and immunological stressors.
PATENT
Product case, WO2008036579 ,
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2008036579
Example 16
3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-7-(l-ethyl-propyl)-2,5-dimethyl- pyrazolo [ 1 ,5 -α]pyrimidine
Under a nitrogen atmosphere dissolve 3-(4-bromo-2-morpholin-4-yl-thiazol-5-yl)-7-(l-ethyl-propyl)-2,5-dimethyl-pyrazolo[l,5-α]pyrimidine (116 mg, 0.25 mmol) in THF (1.5 mL) and chill to -78 0C. Add n-butyl lithium (0.1 mL. 2.5 M in hexane, 0.25 mmol) and stir at -78 0C for 30 min. Add N-chlorosuccinimide (33.4 mg, 0.25 mmol) and stir for another 30 min, slowly warming to room temperature. After stirring overnight, quench the reaction by adding a solution of saturated ammonia chloride and extract with ethyl acetate. Wash the organic layer with brine, dry over sodium sulfate, filter, and concentrate to a residue. Purify the crude material by flash chromatography, eluting with hexanes:dichloromethane: ethyl acetate (5:5:2) to provide the title compound (54 mg). MS (APCI) m/z (35Cl) 420.6 (M+l)+; 1H NMR (400 MHz, CDCl3): 6.44 (s, IH), 3.79 (t, 4H, J=4.8 Hz), 3.63-3.56 (m, IH), 3.47 (t, 4H, J=4.8 Hz), 2.55 (s, 3H), 2.45 (s, 3H), 1.88-1.75 (m, 4H), 0.87 (t, 6H, J=7.5 Hz).
Alternate Preparation from Preparation 6:
Combine 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-α]pyrimidine, (9 g,
26.2 mmol) and 4-chloro-2-morpholino-thiazole (7.5 g, 36.7 mmol) in
dimethylformamide (90 mL) previously degassed with nitrogen. Add cesium carbonate (17.8 g, 55 mmol), copper iodide (250 mg, 1.31 mmol), triphenylphosphine (550 mg, 2.09 mmol) and palladium acetate (117 mg, 0.52 mmol). Heat the mixture to 125 0C for 16 h and then cool to 22 0C. Add water (900 mL) and extract with methyl-?-butyl ether (3 x 200 mL). Combine the organic portions and evaporate the solvent. Purify by silica gel chromatography eluting with hexanes/ethyl acetate (4/1) to afford the title compound (6.4 g, 62%). ES/MS m/z (35Cl) 420 (M+l)+.
Example 16a
3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-7-(l-ethyl-propyl)-2,5-dimethyl- pyrazolo[l,5-α]pyrimidine, hydrochloride
Dissolve 3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-7-(l-ethyl-propyl)-2,5-dimethyl-pyrazolo[l,5-α]pyrimidine (1.40 g, 3.33 mmol) in acetone (10 mL) at 50 0C and cool to room temperature. Add hydrogen chloride (2 M in diethyl ether, 2.0 mL, 4.0 mmol) and stir well in a sonicator. Concentrate the solution a little and add a minimal amount of diethyl ether to crystallize the HCl salt. Cool the mixture in a refrigerator overnight. Add additional hydrogen chloride (2 M in diethyl ether, 2.0 mL, 4.0 mmol) and cool in a refrigerator. Filter the crystalline material and dry to obtain the title compound (1.15 g, 75%). ES/MS m/z (35Cl) 420 (M+l)+; 1H NMR(CDCO): 9.18 (br, IH), 6.86 (s, IH), 3.72 ( m, 4H), 3.49(m, IH), 3.39 (m, 4H), 2.48 (s, 3H), 2.38(s, 3H), 1.79 (m, 4H), 0.79 (m, 6H).
PATENT
US-20200255436
PATENT
WO2019210266
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019210266
claiming the use of CRF-1 antagonists (eg tildacerfont).
PATENT
WO 2010039678
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010039678
EXAMPLES
Example 1 : 7-(l-ethyl-propyl)-3-(‘2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolori ,5-alpyrimidine nthroline
Charge 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (1.03 g, 3.00 mmoles), K3PO4 (1.95 g, 9.00 mmoles), 2,4-dichlorothiazole (0.58 g, 3.75 mmoles), 1,10 phenanthroline (0.05 g, 0.30 mmoles) and anhydrous DMAC (5 mL) to a round bottom flask equipped with a magnetic stir bar, thermal couple and N2 inlet. Degas the yellow heterogeneous reaction mixture with N2 (gas) for 30 min. and then add CuI (0.06 g, 0.30 mmoles) in one portion followed by additional 30 min. degassing with N2 (gas). Stir the reaction mixture at 120 0C for about 6 hr. Cool the reaction mixture to room temperature overnight, add toluene (10 mL) and stir for 1 hr. Purify the mixture through silica gel eluting with toluene (10ml). Extract with 1 M HCl (10 mL), water (10 mL), brine (10 mL) and concentrate under reduced pressure to give a yellow solid. Recrystallize the solid from methanol (5ml) to yield the title compound as a yellow crystalline solid. (0.78 g, 70% yield, >99% pure by LC) MS(ES) = 369 (M+ 1). 1H NMR (CDCl3)= 6.5 (IH, s); 3.6 (IH, m); 2.6 (3H, s); 2.5 (3H, s); 1.9 (4H, m); 0.9 (6H, t).
Example 2: 7-(l-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolol“! ,5-aipyrimidine
Charge 7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (0.37 g, 1.00 mmoles), K2CO3 (0.28 g, 2.00 mmoles) and anhydrous morpholine (3 mL) to a round bottom flask equipped with a magnetic stir bar and N2 inlet. Stir the yellow mixture at 100 0C for about 4 hr., during which time the reaction becomes homogeneous. Cool the reaction mixture to room temperature, add H2O (10 mL) and stir the heterogeneous reaction mixture overnight at room temperature. Collected the yellow solid by filtration, wash with H2O and allowed to air dry overnight to give the crude title compound (391mg). Recrystallize from isopropyl alcohol (3 mL) to yield the title compound as a light yellow crystalline solid (380 mg, 90.6% yield, >99% by LC). MS(ES) = 420 (M+l). 1H NMR (CDCl3)= 6.45 (IH, s); 3.81 (m, 4H); 3.62 (IH, m); 3.50 (m, 4H); 2.6 (3H, s); 2.45 (3 H, s); 1.85 (4H, m); 0.9 (6H, t).
Example 3 :
The reactions of Example 1 are run with various other catalysts, ligands, bases and solvents, which are found to have the following effects on yield of 7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine. (See Tables 1 – 4).
Table 1 : Evaluation of different li ands
(Reactions are carried out in parallel reactors with 1.2 mmol 2,4-dichlorothiazole, 1 mmol 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine, 0.5 mmol CuI, 0.5 mmol ligand and 2.1 mmol Cs2CO3 in 4 mL DMAC. The reactions are degassed under N2 for 30 min. and then heated at between 80 and
1000C overnight under N2. Percent product is measured as the percent of total area under the HPLC curve for the product peak. Longer reaction times are shown in parenthesis) Table 2: Evaluation of various solvents
(Reactions are carried out in parallel reactors with 1.2 mmol 2,4-dichlorothiazole 1 mmol 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine, 0.25 mmol CuI, 0.25 mmol 1,10-phenanthroline and 2.1 mmol Cs2CO3 in 3 mL specified solvent. The reactions are degassed under N2 for 30 minutes and then heated at 1000C overnight under N2. Percent product is measured as the percent of total area under the HPLC curve for the product peak.)
Table 3 : Evaluation of different copper sources
(Reactions are carried out in in parallel reactors with 1 mmol 2,4-dichlorothiazole 1 mmol 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine, 0.05 mmol CuX, 0.01 mmol 1,10-phenanthroline and 3 equivalents K3PO4 in 3 mL DMAC. The reactions are degassed under N2 for 30 minutes and then heated at 1000C overnight under N2. Percent product is measured as the percent of total area under the HPLC curve for the product peak.)
Table 4: Evaluation of various inorganic bases
(Reactions are carried out in in parallel reactors with 1 mmol 2,4-dichlorothiazole 1 mmol 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine, 0.1 mmol CuI, 0.1 mmol 1,10-phenanthroline and 2.1 mmol base and degassed for 30 minutes prior to the addition of 3 mL DMAC. The reactions are degassed under N2 for 10 minutes and then heated at 1000C overnight under N2. Percent product is measured as the percent of total area under the HPLC curve for the product peak.)
Example 4. Use of morpholine both as a reactant and base in 2-MeTHF as solvent.
solvent
7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-ajpyrimidine (15.2 g, 41.16 mmoles) is charged into a 250 mL 3-necked round bottomed flask, followed by addition of 2-MeTHF (61 mL, 4.0 volumes), the yellowish brown slurry is stirred at about 20 0C for 5 min. Then morpholine (19 g, 218.18 mmoles) is added over 2-5 minutes. Contents are heated to reflux and maintained at reflux for 12 hr. The slurry is cooled to 25 0C, followed by addition of 2-MeTHF (53 mL, 3.5 volumes) and water ( 38 mL 2.5 volumes). The reaction mixture is warmed to 40 0C, where upon a homogenous solution with two distinct layers formed. The layers are separated, the organic layer is filtered and concentrated to ~3 volumes at atmospheric pressure. Four volumes 2-propanol (61 mL) are added. The solution is concentrated to ~3 volumes followed by addition of 4 volumes 2-propanol (61 mL), re-concentrated to ~3 volumes, followed by addition of another 6 volumes 2-propanol (91 mL), and refluxed for 15 min. The clear solution is gradually cooled to 75 0C, seeded with 0.45 g 7-(l-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine slurried in 2 mL 2-propanol, rinsed with an additional 2 mL 2-propanol and transferred to a crystallization flask. The slurry is cooled to between 0-5 0C, maintained for 1 hr, filtered and the product rinsed with 2-propanol (30 mL, 2 volumes). The solid is dried at 60 0C in a vacuum oven to afford 16.92 g 7-(l-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine. Purity of product by HPLC assay is 100.00 %. XRPD and DSC data of product is consistant with reference sample. MS(ES) = 420 (M+ 1).
Example 5. Use of morpholine as both reactant and base in 2-propanol as solvent.
7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-ajpyrimidine (11.64 mmoles) is charged into a 100 mL 3 -necked round bottomed flask followed by addition of 2-propanol ( 16 mL, 3.72 volumes). The yellowish brown slurry is stirred at about 20 0C for 5 min. Then morpholine (3.3 g, 37.84 mmoles) is added over 2-5 minutes. Contents are refluxed for 6 hr. The slurry is cooled to 25 0C. 2-Propanol ( 32 mL, 7.44 volumes) and water ( 8.6 mL, 2.0 volumes) are added and the mixture warmed to 70-75 0C, filtered and concentrated to ~ 9 volumes at atmospheric pressure. The clear solution is gradually cooled to 55 0C, seeded with 0.06 g of crystalline 7-(l-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine slurried in 0.5 mL 2-propanol, rinsed with additional 0.5 mL 2-propanol and added to crystallization flask. The slurry is cooled to 0-5 0C, maintained for 1 hr., filtered and the product rinsed with 2-propanol ( 9 mL, 2.1 volumes). Suctioned dried under vacuum at 60 0C to afford 4.6 g of dry 7-(l-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (88.8 % yield, purity by HPLC assay is 99.88 % ). MS(ES) = 420 (M+ 1).
Example 6: 7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolori ,5-alpyrimidine
7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (10 g, 29.17 mmoles), 2, 4-dichlorothiazole (5.2 g , 33.76 mmoles), cesium carbonate(19.9g, 61.07 mmoles) and 1,10-phenanthroline (1 g, 5.5 mmoles) are charged into a 250 mL 3-necked round bottomed flask, followed by 2-MeTHF (36 mL, 3.6 volumes). The reaction mixture is degassed with nitrogen and then evacuated. Cuprous chloride (0.57 g, 5.7 mmoles), DMAC (10 mL, 1 volume) and 2-MeTHF (4 mL, 0.4 volumes) are added in succession. The reaction mixture is degassed with nitrogen and then evacuated. The contents are refluxed for 20 hr. The reaction mixture is cooled to -70 0C and 2-MeTHF (100 mL, 10 volumes) is added. The contents are filtered at ~70 0C and the residual cake is washed with 2-MeTHF (80 mL, 8 volumes) at about 65-72°C. The filtrate is transferred into a separatory funnel and extracted with water. The organic layer is separated and washed with dilute HCl. The resulting organic layer is treated with Darco G60, filtered hot (600C). The filtrate is concentrated at atmospheric pressure to -2.8 volumes. 25 mL 2-propanol is added, followed by re-concentration to -2.8 volumes. An additional 25 mL 2-propanol is added, followed again by re-concentration to -2.8 volumes. Finally, 48 mL 2-propanol is added. The contents are cooled to -7 0C, maintained at -7 0C for 1 hr., filtered and rinsed with 20 mL chilled 2-propanol. Product is suction dried and then vacuum dried at 60 0C to afford 9.41 g 7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (purity of product by HPLC assay is 95.88 %). MS(ES) = 369 (M+ 1).
Example 7. Synthesis of 7-(l-ethyl-propyl)-3-(2, 4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolori,5-a1pyrimidine using 1,4-Dioxane solvent and CuCl catalyst
Add dioxane (9.06X), Cs2CO3 (2.00X), 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (1.0 equivalent), 2,4-dichlorothiazole (0.54 equivalent) to a reactor under N2. Purge the reactor with N2 three times, degas with N2 for 0.5-1 hr., and then add 1,10-phenanthroline (0.3 eq) and CuCl (0.3eq) under N2 , degassing with N2 for 0.5-1 hr. Heat the reactor to 1000C -1100C under N2 . Stir the mixture for 22-24 hr. at 100 0C -1100C. Cool to 10~20°C and add water (10V) and CH3OH (5V), stir the mixture for 1-1.5 hr. at 10~20°C. Filter the suspension, resuspend the wet cake in water, stirr for 1-1.5 hr. at 10~20°C, and filter the suspension again. Charge the wet cake to n-heptane (16V) and EtOAc (2V) under N2. Heat the reactor to 40 °C~500C under N2.
Active carbon (0. IX) is added at 40 °C~500C. The reactor is heated to 55°C~650C under N2 and stirred at 55 °C~650C for 1-1.5 hr. The suspension is filtered at 40~55°C through diatomite (0.4 X). The cake is washed with n-heptane (2.5V). The filtrate is transferred to another reactor. EtOAc (10V) is added and the the organic layer washed with 2 N HCl (10V) three times, followed by washing two times with water (10X, 10V). The organic layer is concentrated to 3-4V below 500C. The mixture is heated to 80-90 0C. The mixture is stirred at this temperature for 40-60 min. The mixture is cooled to 0~5°C, stirred for 1-1.5 hr. at 0~5°C and filtered. The cake is washed with n-heptane (IV) and vacuum dried at 45-500C for 8-10 hr. The crude product is dissolved in 2-propanol (7.5V) under N2, and re-crystallized with 2-propanol. The cake is dried in a vacuum oven at 45°C~50°C for 10-12 hr. (55-80% yield). 1H NMR56.537 (s, IH) 3.591-3.659 (m, IH, J=6.8Hz), 2.593 (s, 3H), 2.512 (s, 3H), 1.793-1.921(m, 4H), 0.885-0.903 (m, 6H).
REFERENCES
1: Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014 Apr;35(2):234-44. doi: 10.1016/j.yfrne.2014.01.001. Epub 2014 Jan 20. Review. PubMed PMID: 24456850; PubMed Central PMCID: PMC4213066.
/////////////tildacerfont, SPR 001, Orphan Drug Status, Congenital adrenal hyperplasia, SPRUCE BIOSCIENCES, PHASE 2
CCC(CC)C1=CC(=NC2=C(C(=NN12)C)C3=C(N=C(S3)N4CCOCC4)Cl)C