VX 759
CAS#: 478025-29-5
Chemical Formula: C22H27NO3S
Molecular Weight: 385.522
478025-29-5
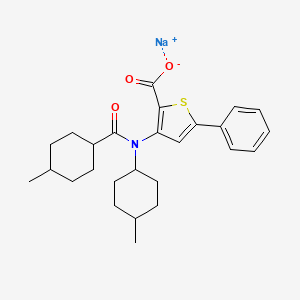
Drug Name:VCH-759Research Code:VX-759; BCH-27759; VCH-759
VX-759; BCH-27759; VCH-759; VX759; BCH27759; VCH759; VX 759; BCH 27759; VCH 759; NNI-1
3-(N-isopropyl-4-methylcyclohexane-1-carboxamido)-5-phenylthiophene-2-carboxylic acid
- 3-[[(trans-4-Methylcyclohexyl)carbonyl](1-methylethyl)amino]-5-phenyl-2-thiophenecarboxylic acid
- 3-[Isopropyl(trans-4-methylcyclohexylcarbonyl)amino]-5-phenylthiophene-2-carboxylic acid
- NNI 1
MOA:NS5B inhibitorIndication:HCV infectionStatus:Phase Ⅱ (Discontinued)Company:Vertex (Originator)
| Molecular Formula | C26H32NNaO3S |
|---|---|
| Synonyms |
VX-759 sodium salt BCP17193 |
| Molecular Weight | 461.6 |
VCH-759 had been in phase II clinical trials by ViroChem Pharma (acquired by Vertex in 2009) for the treatment of HCV infection. However, this research has been discontinued.
Infection with HCV is a major cause of human liver disease throughout the world. In the US, an estimated 4.5 million Americans are chronically infected with HCV. Although only 30% of acute infections are symptomatic, greater than 85% of infected individuals develop chronic, persistent infection. Treatment costs for HCV infection have been estimated at $5.46 billion for the US in 1997. Worldwide over 200 million people are estimated to be infected chronically. HCV infection is responsible for 40-60% of all chronic liver disease and 30% of all liver transplants. Chronic HCV infection accounts for 30% of all cirrhosis, end-stage liver disease, and liver cancer in the U.S. The CDC estimates that the number of deaths due to HCV will minimally increase to 38,000/year by the year 2010.
Due to the high degree of variability in the viral surface antigens, existence of multiple viral genotypes, and demonstrated specificity of immunity, the development of a successful vaccine in the near future is unlikely. Alpha-interferon (alone or in combination with ribavirin) has been widely used since its approval for treatment of chronic HCV infection. However, adverse side effects are commonly associated with this treatment: flu-like symptoms, leukopenia, thrombocytopenia, depression from interferon, as well as anemia induced by ribavirin (Lindsay, K. L. (1997) Hepatology 26 (suppl 1 ): 71 S-77S). This therapy remains less effective against infections caused by HCV genotype 1 (which constitutes -75% of all HCV infections in the developed markets) compared to infections caused by the other 5 major HCV genotypes. Unfortunately, only -50-80% of the patients respond to this treatment (measured by a reduction in serum HCV RNA levels and normalization of liver enzymes) and, of responders, 50-70% relapse within 6 months of cessation of treatment. Recently, with the introduction of pegylated interferon (Peg-IFN), both initial and sustained response rates have improved substantially, and combination treatment of Peg-IFN with ribavirin constitutes the gold standard for therapy. However, the side effects associated with combination therapy and the impaired response in patients with genotype 1 present opportunities for improvement in the management of this disease.
First identified by molecular cloning in 1989 (Choo, Q-L et al (1989) Science 244:359-362), HCV is now widely accepted as the most common causative agent of post-transfusion non A, non-B hepatitis (NANBH) (Kuo, G et al (1989) Science 244:362-364). Due to its genome structure and sequence homology, this virus was assigned as a new genus in the Flaviviridae family. Like the other members of the Flaviviridae, such as flaviviruses (e.g. yellow fever virus and Dengue virus types 1-4) and pestiviruses (e.g. bovine viral diarrhea virus, border disease virus, and classic swine fever virus) (Choo, Q-L et al (1989) Science 244:359-362; Miller, R.H. and R.H. Purcell (1990) Proc. Natl. Acad. Sci. USA 87:2057-2061 ), HCV is an enveloped virus containing a single strand RNA molecule of positive polarity. The HCV genome is approximately 9.6 kilobases (kb) with a long, highly conserved, noncapped 5′ nontranslated region (NTR) of approximately 340 bases which functions as an internal ribosome entry site (IRES) (Wang CY et al ‘An RNA pseudoknot is an essential structural element of the internal ribosome entry site located within the hepatitis C virus 5′ noncoding region’ RNA- A Publication of the RNA Society. 1 (5): 526-537, 1995 JuL). This element is followed by a region which encodes a single long open reading frame (ORF) encoding a polypeptide of -3000 amino acids comprising both the structural and nonstructural viral proteins.
Upon entry into the cytoplasm of the cell, this RNA is directly translated into a polypeptide of -3000 amino acids comprising both the structural and nonstructural viral proteins. This large polypeptide is subsequently processed into the individual structural and nonstructural proteins by a combination of host and virally-encoded proteinases (Rice, CM. (1996) in B.N. Fields, D.M.Knipe and P.M. Howley (eds) Virology 2nd Edition, p931-960; Raven Press, N.Y.). Following the termination codon at the end of the long ORF, there is a 3′ NTR which roughly consists of three regions: an – 40 base region which is poorly conserved among various genotypes, a variable length poly(U)/polypyrimidine tract, and a highly conserved 98 base element also called the “3′ X-tail” (Kolykhalov, A. et al (1996) J. Virology 70:3363-3371 ; Tanaka, T. et al (1995) Biochem Biophys. Res. Commun. 215:744-749; Tanaka, T. et al (1996) J. Virology 70:3307-3312; Yamada, N. et al (1996) Virology 223:255-261 ). The 3′ NTR is predicted to form a stable secondary structure which is essential for HCV growth in chimps and is believed to function in the initiation and regulation of viral RNA replication.
The NS5B protein (591 amino acids, 65 kDa) of HCV (Behrens, S. E. et al (1996) EMBO J. 15:12-22), encodes an RNA-dependent RNA polymerase (RdRp) activity and contains canonical motifs present in other RNA viral polymerases. The NS5B protein is fairly well conserved both intra-typically (-95-98% amino acid (aa) identity across 1 b isolates) and inter-typically (-85% aa identity between genotype 1 a and 1 b isolates). The essentiality of the HCV NS5B RdRp activity for the generation of infectious progeny virions has been formally proven in chimpanzees (A. A. Kolykhalov et al.. (2000) Journal of Virology, 74(4): 2046-2051 ). Thus, inhibition of NS5B RdRp activity (inhibition of RNA replication) is predicted to be useful to treat HCV infection.
Although the predominant HCV genotype worldwide is genotype 1, this itself has two main subtypes, denoted 1a and 1 b. As seen from entries into the Los Alamos HCV database
(www.hcv.lanl.gov) (Table 1 ) there are regional differences in the distribution of these subtypes: while genotype 1 a is most abundant in the United States, the majority of sequences in Europe and Japan are from genotype 1 b.
Table 1
Based on the foregoing, there exists a significant need to identify synthetic or biological compounds for their ability to inhibit replication of both genotype 1 a and genotype 1 b of HCV.
PATENT
WO 2002100851
WO 2007071434
PATENT
WO 2009000818
Compound A
5-Phenyl-3-[[(frans-4-methylcyclohexyl)carbonyl](1-methylethyl)amino]-2-thiophenecarboxylic acid
To a mixture of methyl S-^trans^-methylcyclohexyOcarbonylKI-methylethy^aminol-S-phenyl-2-thiophenecarboxylate (Intermediate 31 ) (390 mg) in THF/MeOH/water (3:2:1, vol/vol, 40 ml. total) was added lithium hydroxide monohydrate (246 mg). The mixture was stirred at room temperature for 20 hours, the solvents removed in vacuo, and the residue partitioned between water (40 ml.) and ethyl acetate (40 ml_). The organic layer was dried
(Na2SC>4), evaporated and triturated with ether to give the title compound.
MS calcd for (C22H27NO3S+ H)+: 356
MS found (electrospray): (M+H)+ =356
Compounds A, B, C and D may be made according to the processes described in WO2002/100851 or as described hereinabove.
Structures of Compounds A, B, C and D are shown below for the avoidance of doubt.
The compounds of Formula (I) which have been tested demonstrate a surprisingly superior potency as HCV polymerase inhibitors, as shown by the IC5O values in the cell-based assays across both of the 1 a and 1 b genotypes of HCV, compared to Compounds A, B, C and D. Accordingly, the compounds of Formula (I) are of great potential therapeutic benefit in the treatment and prophylaxis of HCV.
PAPER
Bioorganic & Medicinal Chemistry Letters (2016), 26(18), 4536-4541.
https://www.sciencedirect.com/science/article/abs/pii/S0960894X16300427
| Application Id | Application Number | Application Date | Country | Title |
| US333830037 | 16610899 | 04.05.2018 | US | IDENTIFICATION AND TARGETED MODULATION OF GENE SIGNALING NETWORKS |
| US77274496 | 13661508 | 26.10.2012 | US | COMPOUNDS AND METHODS FOR THE TREATMENT OR PREVENTION OF FLAVIVIRUS INFECTIONS |
| US73506396 | 13172477 | 29.06.2011 | US | Thiophene analogues for the treatment or prevention of flavivirus infections |
| EP29858255 | 10185737 | 11.06.2002 | EP | Thiophene derivatives as antiviral agents for flavivirus infection |
| US73326585 | 13081780 | 07.04.2011 | US | Compounds and methods for the treatment or prevention of <i>Flavivirus </i>infections |
| JP272602128 | 2010103706 | 28.04.2010 | JP | COMPOUND AND METHOD FOR TREATMENT OR PREVENTION OF FLAVIVIRUS INFECTION |
| EP11167270 | 09167203 | 11.06.2002 | EP | Thiophene derivatives as antiviral agents for flavivirus infection |
| CN83819886 | 200910139375.5 | 11.06.2002 | CN | Thiophene derivatives as antiviral agents for flavivirus infection |
| US42846304 | 12097840 | 20.12.2006 | US | Antiviral 2-Carboxy-Thiophene Compounds |
| JP272180287 | 2008546276 | 20.12.2006 | JP | 抗ウイルス性2-カルボキシ-チオフェン化合物 |
| WO2007071434 | PCT/EP2006/012442 | 20.12.2006 | WO | ANTIVIRAL 2-CARBOXY-THIOPHENE COMPOUNDS |
| US41429134 | 11042442 | 26.01.2005 | US | Compounds and methods for the treatment or prevention of <i>Flavivirus </i>infections |
| CN82792692 | 02815768.0 | 11.06.2002 | CN | Thiophene derivatives used as antiviral agent against flavivirus infections |
| JP270324690 | 2003503618 | 11.06.2002 | JP | FLAVIVIRUS感染の治療または予防のための化合物および方法 |
| EA95398282 | 200400022 | 11.06.2002 | EA | COMPOUNDS AND METHODS FOR THE TREATMENT OR PREVENTION OF FLAVIVIRUS INFECTIONS |
| US40367952 | 10166031 | 11.06.2002 | US | Compounds and methods for the treatment or prevention of Flavivirus infections |
| KR588271 | 1020037016240 | 11.12.2003 | KR | THIOPHENE DERIVATIVES AS ANTIVIRAL AGENTS FOR FLAVIVIRUS INFECTION |
| EP14092312 | 02742563 | 11.06.2002 | EP | THIOPHENE DERIVATIVES AS ANTIVIRAL AGENTS FOR FLAVIVIRUS INFECTION |
| WO2002100851 | PCT/CA2002/000876 | 11.06.2002 | WO | THIOPHENE DERIVATIVES AS ANTIVIRAL AGENTS FOR FLAVIVIRUS INFECTION |
////////////////VX-759, BCH-27759, VCH-759, VX759, BCH27759, VCH759, VX 759, BCH 27759, VCH 759, NNI-1
O=C(C1=C(N(C(C2CCC(C)CC2)=O)C(C)C)C=C(C3=CC=CC=C3)S1)O