
Zydus receives approval from USFDA to initiate Phase II clinical studies of Saroglitazar Magnesium in patients with Primary Biliary Cholangitis (PBC) Read more: https://goo.gl/eugRnZ #ZydusAnnouncement
Ahmedabad, India, February 23, 2017

Zydus Cadila, a research-driven, global healthcare provider, today announced that the USFDA has approved the group’s plans to initiate a Phase 2 clinical trial of Saroglitazar Magnesium (Mg) in patients with Primary Biliary Cholangitis (PBC) of the liver. This randomized, double-blind Phase 2 trial will evaluate Saroglitazar Magnesium 2mg and 4 mg Vs. Placebo.
Speaking on the development, Mr. Pankaj R. Patel, Chairman and Managing Director, Zydus Cadila said, “We are very thankful to the USFDA for their timely and useful feedback on the clinical trial designs of Saroglitazar Mg in patients with Primary Biliary Cholangitis (PBC). This development underlines our commitment to bridging unmet healthcare needs with innovative therapies.”
Primary Biliary Cholangitis (PBC) is a liver disease, caused due to progressive destruction of the bile ducts in the liver which leads to reduction of bile flow – a condition referred to as cholestasis. PBC is often discovered incidentally due to abnormal results on routine liver blood tests. Progression of PBC leads to symptoms of cirrhosis like yellowing of the skin, swelling of legs and feet (edema), ascites, internal bleeding (varices) and thinning of the bones (osteoporosis). The buildup of toxic bile in the liver leads to liver inflammation and fibrosis which can progress to cirrhosis. People with cirrhosis are at increased risk of hepatocellular carcinoma or liver cancer, which is a leading cause of liver transplants or death.
With an increasing number of people being affected by PBC which can lead to progressive cholestasis and even turn fatal, there is a pressing need to develop therapies which help to achieve an adequate reduction in alkaline phosphotase (ALP) or bilirubin and bring in better tolerance and efficacy.
About Lipaglyn™ Lipaglyn™ is a prescription drug authorized for sale in India only. Lipaglyn™ was launched in India during Sept 2013 for the treatment of Hypertriglyceridemia and Diabetic Dyslipidemia in Patients with Type 2 Diabetes not controlled by statins. Saroglitazar Mg is an investigational new drug with the USFDA, and is currently under clinical investigation for three significant unmet medical needs in the United States – Primary Biliary Cholangitis (PBC), Non-alcoholic Steatohepatitis (NASH) and Severe Hypertriglyceridemia (TG>500).
About Zydus Zydus Cadila is an innovative, global healthcare provider that discovers, develops, manufactures and markets a broad range of healthcare therapies, including small molecule drugs, biologic therapeutics and vaccines. The group employs over 19,500 people worldwide, including 1200 scientists engaged in R & D, and is dedicated to creating healthier communities globally. For more information, please visit www.zyduscadila.com
http://zyduscadila.com/wp-content/uploads/2017/02/USFDA-approval-for-clinical-trial-of-Saro-Mg.pdf
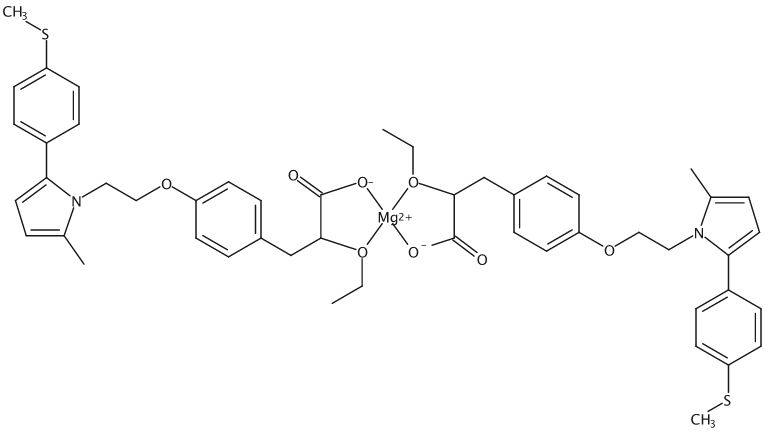

Saroglitazar magnesium
CAS: 1639792-20-3
Molecular Formula, 2C25-H28-N-O4-S.Mg,
Molecular Weight, 901.4354
Magnesium, bis((alphaS)-alpha-(ethoxy-kappaO)-4-(2-(2-methyl-5-(4-(methylthio)phenyl)-1H-pyrrol-1-yl)ethoxy)benzenepropanoato-kappaO)-, (T-4)-
(2S)-2-Ethoxy-3-(4-(2-(2-methyl-5-(4-(methylsulfanyl)phenyl)-1H-pyrrol-1-yl(ethoxy)phenyl)propanoic acid, magnesium salt (2:1)

DR RANJIT DESAI
ZYDUS

//////////Zydus, USFDA, Phase II, clinical studies, Saroglitazar Magnesium, Primary Biliary Cholangitis, (PBC)
[Mg+2].CCO[C@@H](Cc1ccc(OCCn2c(C)
















