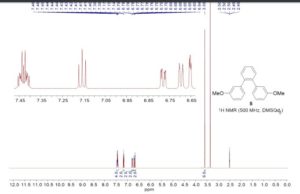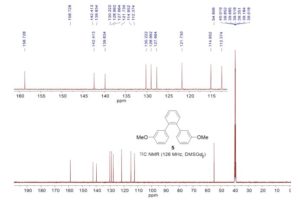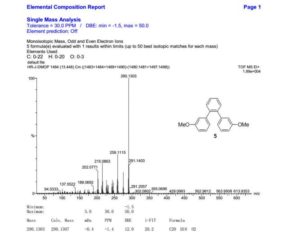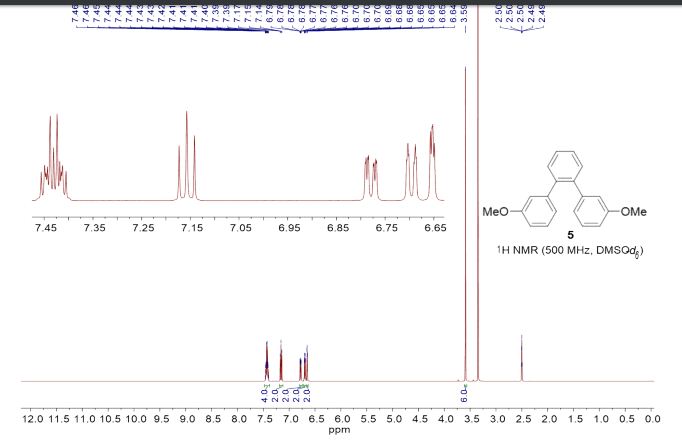
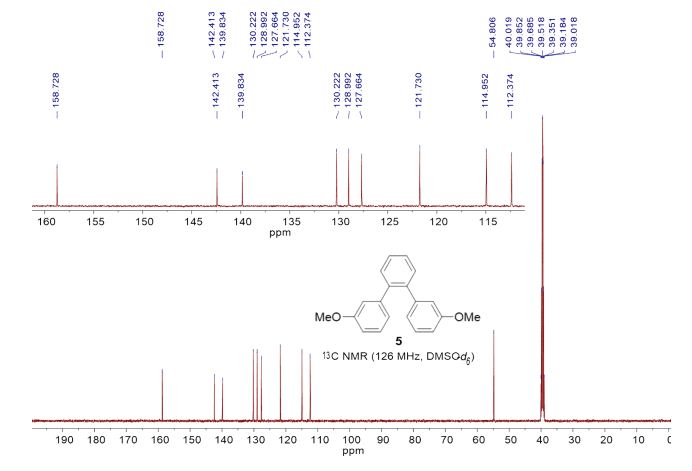

1,2-Bis(3-methoxyphenyl)benzene (5)
Efficient and Practical Synthesis of Electron Transport Material and Its Key Intermediate
Abstract
An efficient and practical synthesis of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)triphenylene 4 from two cheap commodity chemicals in five steps with a total yield of 48.6% was developed. This process had been successfully applied in the synthesis of electron transport material (ETM) BPyTP-2 in the gram scale with a total yield of 47.2%. This practical development of the key intermediate 4 opens a door in its further application in the synthesis of other triphenylene-based ETMs and host materials in the materials field.
/////////////http://pubs.acs.org/doi/10.1021/acs.oprd.7b00280
http://pubs.acs.org/doi/suppl/10.1021/acs.oprd.7b00280/suppl_file/op7b00280_si_001.pdf