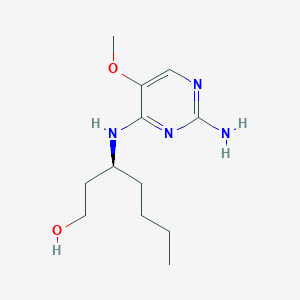
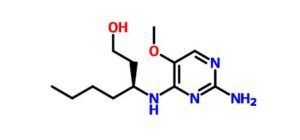
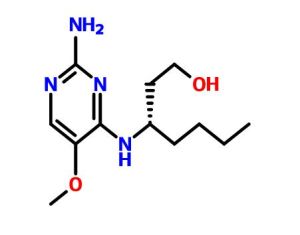
(3S)-3-[(2-amino-5-methoxypyrimidin-4-yl)amino]heptan-1-ol
cas 1402802-45-2
JNJ ?
WO 2012136834, WO 2014053595
for the treatment of viral infections
| Molecular Formula: | C12H22N4O2 |
|---|---|
| Molecular Weight: | 254.32868 g/mol |

(3S)-3-[(2-amino-5-methoxypyrimidin-4-yl)amino]heptan-1-ol

Mark Hicken, Managing Director UK & Ireland, Janssen Ltd; Maggie Throup MP, Health Select Committee; Richard Mason, Head of Innovation, Johnson and Johnson; Dr Mark Lloyd Davies, Senior Director Government Affairs and Policy UK and Ireland, Johnson & Johnson
To the use of pyrimidine derivatives in the treatment of viral infections, immune or inflammatory disorders, whereby the modulation, or agonism, of toll-like-receptors (TLRs) is involved. Toll-Like Receptors are primary transmembrane proteins characterized by an extracellular leucine rich domain and a cytoplasmic extension that contains a conserved region. The innate immune system can recognize pathogen-associated molecular patterns via these TLRs expressed on the cell surface of certain types of immune cells. Recognition of foreign pathogens activates the production of cytokines and upregulation of co-stimulatory molecules on phagocytes. This leads to the modulation of T cell behaviour.
It has been estimated that most mammalian species have between ten and fifteen types of Toll-like receptors. Thirteen TLRs (named TLR1 to TLR13) have been identified in humans and mice together, and equivalent forms of many of these have been found in other mammalian species. However, equivalents of certain TLR found in humans are not present in all mammals. For example, a gene coding for a protein analogous to TLR10 in humans is present in mice, but appears to have been damaged at some point in the past by a retrovirus. On the other hand, mice express TLRs 11, 12, and 13, none of which are represented in humans. Other mammals may express TLRs which are not found in humans. Other non-mammalian species may have TLRs distinct from mammals, as demonstrated by TLR14, which is found in the Takifugu pufferfish. This may complicate the process of using experimental animals as models of human innate immunity.
For detailed reviews on toll-like receptors see the following journal articles. Hoffmann, J. A., Nature, 426, p 33-38, 2003; Akira, S., Takeda, K., and Kaisho, T., Annual Rev. Immunology, 21, p 335-376, 2003; Ulevitch, R. J., Nature Reviews: Immunology, 4, p 512-520, 2004.
Compounds indicating activity on Toll-Like receptors have been previously described such as purine derivatives in WO 2006/117670, adenine derivatives in WO 98/01448 and WO 99/28321, and pyrimidines in WO 2009/067081. However, there exists a strong need for novel Toll-Like receptor modulators having preferred selectivity, higher potency, higher metabolic stability, and an improved safety profile compared to the compounds of the prior art.
In the treatment of certain viral infections, regular injections of interferon (IFNα) can be administered, as is the case for hepatitis C virus (HCV), (Fried et. al. Peginterferon-alfa plus ribavirin for chronic hepatitis C virus infection, N Engl J Med 2002; 347: 975-82). Orally available small molecule IFN inducers offer the potential advantages of reduced immunogenicity and convenience of administration. Thus, novel IFN inducers are potentially effective new class of drugs for treating virus infections. For an example in the literature of a small molecule IFN inducer having antiviral effect see De Clercq, E.; Descamps, J.; De Somer, P. Science 1978, 200, 563-565.
IFNα is also given in combination with other drugs in the treatment of certain types of cancer (Eur. J. Cancer 46, 2849-57, and Cancer Res. 1992, 52, 1056). TLR 7/8 agonists are also of interest as vaccine adjuvants because of their ability to induce pronounced Th1 response (Hum. Vaccines 2010, 6, 1-14; Hum. Vaccines 2009, 5, 381-394).
SYNTHESIS
50d
patent
WO 2014053595
https://www.google.com/patents/WO2014053595A1?cl=en
Experimental Section.
B
Into a 50 ml_ vial equipped with a magnetic stir bar was placed 2,4-dichloro-5- methoxypyrimidine (2.0 g, 1 1.7 mmol), and acetonitrile (20 ml_), diisopropylethylamine (3.02 g, 23.4 mmol) and (S)-3-aminoheptanol (4.59 g, 35.1 mmol). The reaction mixture was allowed to stir 15 hours at room temperature. The solvents were removed under reduced pressure. The crude was purified via silica gel column chromatography using a dichloromethane to 10% methanol in dichloromethane gradient. The best fractions were pooled and the solvents were removed under reduced pressure to afford a white solid, B.
B C
To a thick wall glass vial equipped with a magnetic stir bar was added B (1 g, 3.66 mmol), NH3 (10 ml_, aq.), ammonium bicarbonate (3.34 g, 42.3 mmol) and copper(l) oxide (121 mg, 0.85 mmol). The vial was sealed and placed into an oil bath and heated to 150°C for 15 hours. The reaction mixture was extracted with dichloro- methane (3 x 25 ml_), the organic layers were pooled and dried over magnesium sulfate. The solids were removed by filtration and the solvents of the filtrate were removed under reduced pressure. Crude C was purified via HPLC.
c 1
C (463 mg, 1.82 mmol) was dissolved in THF (13 ml_) and cooled to -78°C. NaH (145 mg, 3.64 mmol, a 60% dispersion in mineral oil) was added in one portion and stirred at -78°C for 30 minutes. Isobutyryl chloride (389 μΙ_, 3.64 mmol) was added dropwise at -78°C and stirred for 10 minutes. The cooling bath was removed and the mixture was allowed to reach room temperature. The mixture was stirred at room temperature for 30 minutes. The mixture was quenched with water and concentrated in vacuo. The residue was purified by HPLC (RP Vydac Denali C18 10 μηι, 200 g, 5 cm, mobile phase 0.25% NH4HCO3 solution in water, acetonitrile), the desired fractions were collected, and the solvents were removed under reduced pressure to afford the pure product.
LC-MS m/z = 395 (M+H), Retention time 1.1 minutes, LC method A.
1H NMR (400 MHz, DMSO-d6) δ ppm 0.83 (t, J=6.90 Hz, 3 H) 0.98 – 1.07 (m, 12 H) 1.16 – 1.35 (m, 4 H) 1.44 – 1.62 (m, 2 H) 1.84 (q, J=6.78 Hz, 2 H) 2.45 (spt, J=7.00 Hz, 1 H) 2.96 (br. s., 1 H) 3.80 (s, 3 H) 3.92 – 4.07 (m, 2 H) 4.18 – 4.31 (m, 1 H) 6.69 (d, J=9.03 Hz, 1 H) 7.60 (s, 1 H) 9.49 (s, 1 H).
Synthetic scheme for the preparation of A
Preparati
valeraldehyde A2
To a solution of valeraldehyde (43 g, 500 mmol) in THF (1 L) was added A1 (200 g, 532 mmol) and the reaction mixture was stirred for 16 hours at room temperature. The solvents were evaporated and the residue was diluted in petroleum ether and filtered. The solvents of the filtrate were removed under reduced pressure and the residue was purified by silica chromatography using a petroleum ether to 3% ethyl acetate in petroleum ether gradient to give A2 (90 g) as a colorless oil.
1H NMR (400 MHz, CDCI3): δ ppm 6.81 -6.77 (m, 1 H), 5.68-5.64 (td, J=1.2Hz, 15.6 Hz, 1 H), 2.11-2.09 (m, 2H), 1.41 (s, 9H), 1.38-1.26 (m, 4H), 0.85-0.81 (t, J=7.2Hz, 3H).
/7-butyl lithium (290 mL, 725 mmol) was added to a stirred solution of A3 (165 g, 781 mmol) in THF (800 mL) at -78°C. The reaction mixture was stirred for 30 minutes then A2 (90 g, 488.4 mmol) in THF (400 mL) was added and the reaction was stirred for 2 hours at -78°C. The mixture was quenched with sat., aq. NH4CI solution and warmed to room temperature. The product was partitioned between ethyl acetate and water. The organic phase was washed with brine, dried and evaporated. The residue was purified by column chromatography eluting with 5% ethyl acetate in petroleum ether to afford a colorless oil, A4 (132 g).
1H NMR (400 MHz, CDCI3): δ ppm 7.36-7.16 (m, 10H), 3.75-3.70 (m, 2H), 3.43-3.39 (d, J=15.2Hz, 1 H), 3.33-3.15 (m, 1 H), 1.86-1.80 (m, 2H), 1.47-1.37 (m, 2H), 1.32 (s, 9H), 1.26-1.17 (m, 7H), 0.83-0.79 (t, J=7.2Hz, 3H).
Preparatio
A4 (130 g, 328 mmol) was dissolved in THF (1.5 L) and LAH (20 g, 526 mmol) was added at 0°C in small portions. The resulting mixture was stirred at the same temperature for 2 hours and then allowed to warm to room temperature. The mixture was quenched with a sat. aq. NH4CI solution. The product was partitioned between ethyl acetate and water. The organic phase was washed with brine, dried and evaporated. The combined organic layers were dried over sodium sulfate, the solids were removed via filtration and concentrated to afford crude A5 (100 g), which was used in the next step without further purification.
1H NMR (400 MHz, CDCI3): δ ppm 7.33-7.14 (m, 10H), 3.91 -3.86 (m, 1 H), 3.80-3.77 (d, J=13.6Hz, 1 H), 3.63-3.60 (d, J=13.6Hz, 1 H), 3.43-3.42 (m, 1 H), 3.15-3.10 (m, 1 H), 2.70-2.63 (m, 2H), 1.65-1.28 (m, 10H), 0.89-0.81 (m, 3H).
Preparation of A
A5 A
A solution of A5 (38 g, 116.75 mmol) and 10% Pd/C in methanol (200 ml_) was hydrogenated under 50 PSI hydrogen at 50°C for 24 hours. The reaction mixture was filtered and the solvent was evaporated to give A.
1H NMR (400 MHz, DMSO-d6): δ ppm 8.04 (s, 3H), 3.60-3.49 (m, 2H), 3.16-3.15 (m, 1 H), 1.71-1.67 (m, 2H), 1.60-1.55 (m, 2H), 1.33-1.26 (m, 4H), 0.90-0.87 (t, J=6.8Hz, 3H).
Analytical Method.
Compounds 1-8 in the table below were characterized by LC-MS according to the following LC-MS method.
Reverse phase UPLC (Ultra Performance Liquid Chromatography) was carried out on a bridged ethylsiloxane/silica hybrid (BEH) C18 column (1.7 μηι, 2.1 x 50 mm; Waters Acquity) with a flow rate of 0.8 ml/min. Two mobile phases (10 mM ammonium acetate in H20/acetonitrile 95/5; mobile phase B: acetonitrile) were used to run a gradient condition from 95 % A and 5 % B to 5 % A and 95 % B in 1.3 minutes and hold for 0.7 minutes. An injection volume of 0.75 μΙ_ was used. Cone voltage was 30 V for positive ionization mode and 30 V for negative ionization mode.
Paper

Toll-like receptor (TLR) 7 and 8 agonists can potentially be used in the treatment of viral infections and are particularly promising for chronic hepatitis B virus (HBV) infection. An internal screening effort identified a pyrimidine Toll-like receptor 7 and 8 dual agonist. This provided a novel alternative over the previously reported adenine and pteridone type of agonists. Structure–activity relationship, lead optimization, in silico docking, pharmacokinetics, and demonstration of ex vivo and in vivo cytokine production of the lead compound are presented.
Novel Pyrimidine Toll-like Receptor 7 and 8 Dual Agonists to Treat Hepatitis B Virus
(S)-3-((2-Amino-5-methoxypyrimidin-4-yl)amino)heptan-1-ol (50d)
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015274676 | 2015-10-01 | ACYLAMINOPYRIMIDINE DERIVATIVES FOR THE TREATMENT OF VIRAL INFECTIONS AND FURTHER DISEASES |
| US2014045849 | 2014-02-13 | PYRIMIDINE DERIVATIVES FOR THE TREATMENT OF VIRAL INFECTIONS |
| WO2006053109A1 * | Nov 10, 2005 | May 18, 2006 | Synta Pharmaceuticals Corp. | Heteroaryl compounds |
| WO2012136834A1 * | Apr 10, 2012 | Oct 11, 2012 | Janssen R&D Ireland | Pyrimidine derivatives for the treatment of viral infections |
| US5434157 * | Jan 21, 1993 | Jul 18, 1995 | The Upjohn Company | 6-aryl pyrimidine compounds and method for treating viral infections and inducing interferon production |
| Reference | ||
|---|---|---|
| 1 | * | ANGELICA M BELLO ET AL: “De Novo Design of Nonpeptidic Compounds Targeting the Interactions between Interferon-a and its Cognate Cell Surface Receptor“, JOURNAL OF MEDICINAL CHEMISTRY,, vol. 51, no. 9, 1 January 2008 (2008-01-01), pages 2734 – 2723, XP008157384, DOI: 10.1021/JM701182Y |
| 2 | DE CLERCQ, E.; DESCAMPS, J.; DE SOMER, P., SCIENCE, vol. 200, 1978, pages 563 – 565 | |
| 3 | FRIED: “Peginterferon-alfa plus ribavirin for chronic hepatitis C virus infection“, N ENGL J MED, vol. 347, 2002, pages 975 – 82 | |
/////////////// treatment of viral infections, Pyrimidine Toll-like Receptor 7, Toll-like Receptor 8 Dual Agonists, Treat Hepatitis B Virus, PRECLINICAL, 1402802-45-2, JNJ ?
n1c(nc(c(c1)OC)N[C@@H](CCCC)CCO)N