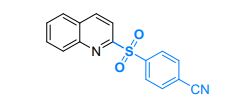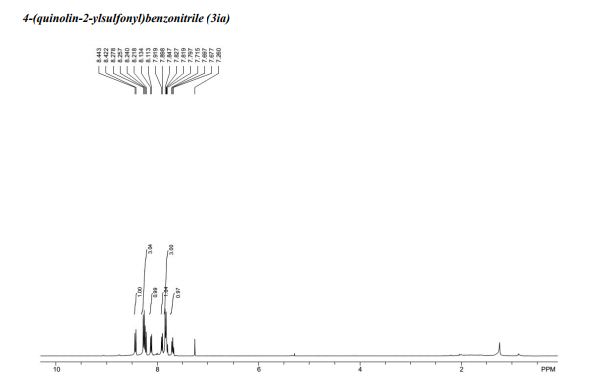
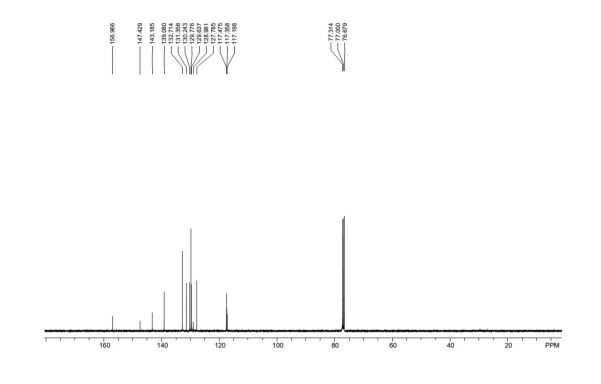
4-(quinolin-2-ylsulfonyl)benzonitrile (3ia)5
1H NMR (400 MHz, CDCl3): = 8.43 (d, J = 8.4 Hz, 1 H), 8.28 – 8.22 (m, 3 H), 8.12 (d, J = 8.4 Hz, 1 H), 7.91 (d, J = 8.4 Hz, 1 H), 7.85 – 7.80 (m, 3 H), 7.70 (t, J = 8.0 Hz, 1 H); 13C NMR (100 MHz, CDCl3): δ = 157.0, 147.4, 143.2, 139.1, 132.7, 131.4, 130.2, 129.8, 129.6, 129.0, 127.8, 117.5, 117.4, 117.2.
Base-free, ultrasound accelerated one-pot synthesis of 2-sulfonylquinolines on water
Abstract
Without employing any base and organic solvent, an economical, practical and eco-friendly protocol has been developed for the one-pot synthesis of various functionalized 2-sulfonylquinolines from easily accessible starting materials on water under open-air conditions. Compared with conventional heating conditions, the use of ultrasound techniques not only improves the reaction efficiency and enhances the reaction rate but also minimizes the side reactions. This process has a broad substrate scope, mild reaction conditions, good yields, excellent chemo- and regioselectivity, operational simplicity, ease of scale-up and high energy efficiency. In addition, the process is remarkably greener than previous routes with an atom economy of 70.7%, E-factor of 1.17 and eco-scale score of 71.
//////////////////http://www.rsc.org/suppdata/c7/gc/c7gc02304a/c7gc02304a1.pdf