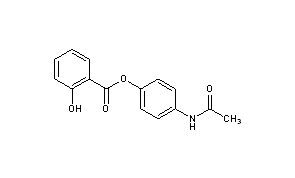

Acetaminosalol
- Molecular FormulaC15H13NO4
- Average mass271.268 Da

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
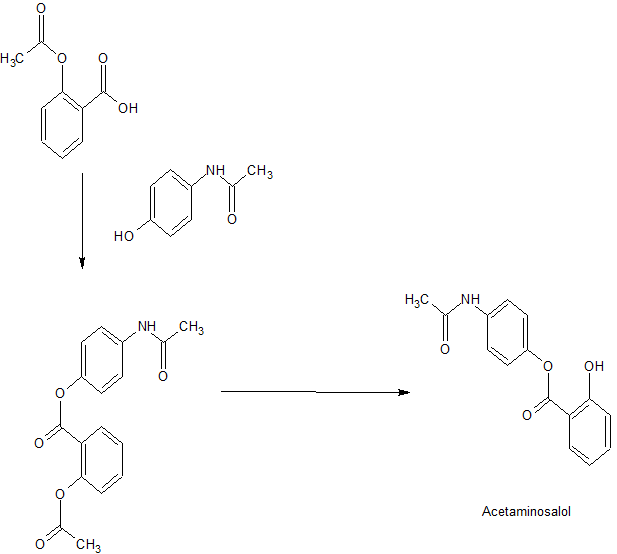
Acetaminosalol is an organic compound with the chemical formula C15H13NO4.
It is an esterification product of salicylic acid and paracetamol. It was marketed by Bayer under the brand name Salophen as an analgesic in the late 19th and early 20th centuries.
Action and uses
In a warm alkaline solution acetaminosalol is broken up into salicylic acid and paracetamol. It is decomposed in the intestines, even when given as an injection. It was used as a substitute for salicylic acid in acute rheumatism, and as an intestinal antiseptic. It was similarly effective and much safer than salol, another intestinal antiseptic commonly used at the time. The fact that it is tasteless renders it easy to administer.
 |
|
| Names | |
|---|---|
| Preferred IUPAC name
4-Acetamidophenyl 2-hydroxybenzoate
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.875 |
| EC Number |
|
| MeSH | Salophen |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C15H13NO4 | |
| Molar mass | 271.272 g·mol−1 |
| Density | 1.327 g cm−3 |
| log P | 2.562 |
| Acidity (pKa) | 7.874 |
| Basicity (pKb) | 6.123 |
| Hazards | |
| Flash point | 241.9 °C (467.4 °F; 515.0 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
///////////////Acetaminosalol, nalgesic , Anti-inflammatory, Salicylic Acid Derivatives, Antipyretic, ацетаминосалол , أسيتامينوسالول , 醋氨沙洛 ,















