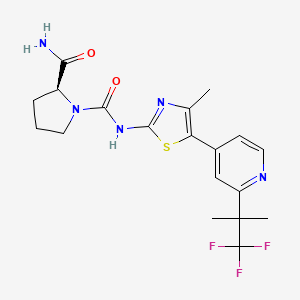
Alpelisib
| Chemical Names: | Alpelisib; CAS 1217486-61-7; BYL-719; BYL719; UNII-08W5N2C97Q; BYL 719 |
|---|---|
| Molecular Formula: | C19H22F3N5O2S |
| Molecular Weight: | 441.473 g/mol |
- alpelisib
- 1217486-61-7
- BYL-719
- BYL719
- UNII-08W5N2C97Q
- BYL 719
- Alpelisib (BYL719)
- (S)-N1-(4-Methyl-5-(2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl)thiazol-2-yl)pyrrolidine-1,2-dicarboxamide
- NVP-BYL719
Alpelisib is an orally bioavailable phosphatidylinositol 3-kinase (PI3K) inhibitor with potential antineoplastic activity. Alpelisib specifically inhibits PI3K in the PI3K/AKT kinase (or protein kinase B) signaling pathway, thereby inhibiting the activation of the PI3K signaling pathway. This may result in inhibition of tumor cell growth and survival in susceptible tumor cell populations. Activation of the PI3K signaling pathway is frequently associated with tumorigenesis. Dysregulated PI3K signaling may contribute to tumor resistance to a variety of antineoplastic agents.


(S)-pyrrolidine-l,2-dicarboxylic acid 2-amide l-(4-methyl-5-[2-(2,2,2-trifluoro-l,l- dimethyl-ethyl)-pyridin-4-yl]-thiazol-2-yl)-amidei hereafter referred to as compound I,
is an alpha-selective phosphatidylinositol 3 -kinase (PI3K) inhibitor. Compound I was originally described in WO 2010/029082, wherein the synthesis of its free base form was described. There is a need for additional solid forms of compound I, for use in drug substance and drug product development. It has been found that new solid forms of compound I can be prepared as one or more polymorph forms, including solvate forms. These polymorph forms exhibit new physical properties that may be exploited in order to obtain new pharmacological properties, and that may be utilized in drug substance and drug product development. Summary of the Invention
In one aspect, provided herein is a crystalline form of the compound of formula I, or a solvate of the crystalline form of the compound of formula I, or a salt of the crystalline form of the compound of formula I, or a solvate of a salt of the crystalline form of the compound of formula I. In one embodiment, the crystalline form of the compound of formula I has the polymorph form SA, SB, Sc, or SD.
In another aspect, provided herein is a pharmaceutical composition comprising a crystalline compound of formula I. In one embodiment of the pharmaceutical composition, the crystalline compound of formula I has the polymorph form SA, SB,Sc, or So.
In another aspect, provided herein is a method for the treatment of disorders mediated by PI3K, comprising administering to a patient in need of such treatment an effective amount of a crystalline compound of formula I, particularly SA, SB, SC,or SD .
In yet another aspect, provided herein is the use of a crystalline compound of formula I, particularly SA, SB, SC, or SD, for the preparation of a medicament for the treatment of disorders mediated by PI3K.
In still another aspect, provided herein is a method for the treatment of disorders selected from benign or malignant tumor; a cancer selected from sarcoma; lung; bronchus; prostate; breast (including sporadic breast cancers and sufferers of Cowden disease);
pancreas; gastrointestinal cancer; colon; rectum; colon carcinoma; colorectal adenoma;
thyroid; liver; intrahepatic bile duct; hepatocellular; adrenal gland; stomach; gastric; glioma; glioblastoma; endometrial; melanoma; kidney; renal pelvis; urinary bladder; uterine corpus; uterine cervix; vagina; ovary; multiple myeloma; esophagus; a leukaemia; acute myelogenous leukemia; chronic myelogenous leukemia; lymphocytic leukemia; myeloid leukemia; brain; a carcinoma of the brain; oral cavity and pharynx; larynx; small intestine; non-Hodgkin lymphoma; melanoma; villous colon adenoma; a neoplasia; a neoplasia of epithelial character; lymphomas; a mammary carcinoma; basal cell carcinoma; squamous cell carcinoma; actinic keratosis; tumor diseases, including solid tumors; a tumor of the neck or head; polycythemia vera; essential thrombocythemia; myelofibrosis with myeloid metaplasia; and Walden stroem disease; as well as polycythemia vera, essential thrombocythemia, myelofibrosis with myeloid metaplasia, asthma, COPD, ARDS, Loffler’s syndrome, eosinophilic pneumonia, parasitic (in particular metazoan) infestation (including tropical eosinophilia), bronchopulmonary aspergillosis, polyarteritis nodosa (including Churg-Strauss syndrome), eosinophilic granuloma, eosinophil-related disorders affecting the airways occasioned by drug-reaction, psoriasis, contact dermatitis, atopic dermatitis, alopecia areata, erythema multiforme, dermatitis herpetiformis, scleroderma, vitiligo, hypersensitivity angiitis, urticaria, bullous pemphigoid, lupus erythematosus, pemphisus, epidermolysis bullosa acquisita, autoimmune haematogical disorders (e.g., haemolytic anaemia, aplastic anaemia, pure red cell anaemia and idiopathic thrombocytopenia), systemic lupus erythematosus, polychondritis, scleroderma, Wegener granulomatosis, dermatomyositis, chronic active hepatitis, myasthenia gravis, Steven-Johnson syndrome, idiopathic sprue, autoimmune inflammatory bowel disease (e.g., ulcerative colitis and Crohn’s disease), endocrine opthalmopathy, Grave’s disease, sarcoidosis, alveolitis, chronic hypersensitivity pneumonitis, multiple sclerosis, primary biliary cirrhosis, uveitis (anterior and posterior), interstitial lung fibrosis, psoriatic arthritis, glomerulonephritis, cardiovascular diseases, atherosclerosis, hypertension, deep venous thrombosis, stroke, myocardial infarction, unstable angina, thromboembolism, pulmonary embolism, thrombolytic diseases, acute arterial ischemia, peripheral thrombotic occlusions, and coronary artery disease, reperfusion injuries, retinopathy, such as diabetic retinopathy or hyperbaric oxygen-induced retinopathy, and conditions characterized by elevated intraocular pressure or secretion of ocular aqueous humor, such as glaucoma, comprising administering to a patient in need of such treatment an effective amount of the crystalline compound of formula I, particularly polymorph forms SA, SB, SC, or SD-
In another aspect, provided herein is the use of the crystalline compound of formula I, particularly polymorph forms SA, SB, SC, or SD for the preparation of a medicament for the treatment of the disorders listed above. Brief Description of the Drawings
Figure I depicts the X-ray powder diffraction pattern of polymorph form A. Figure II depicts the FT-IR spectrum of polymorph form A. Figure III depicts the differential scanning calorimetry thermogram of polymorph form A. Figure IV depicts the X-ray powder diffraction pattern of polymorph form SA- Figure V depicts the X-ray powder diffraction pattern of polymorph form SB. Figure VI depicts the X-ray powder diffraction pattern of polymorph form Sc. Figure VII depicts the X-ray powder diffraction pattern of polymorph form SD.
Scheme 2. Synthesis of (S)-Pyrrolidine-1.2-dicarboxylic acid 2-amide l-((4-methyl-5-r2- (2,2,2-trifluoro- 1 , 1 -dimethyl-ethyl -pyridin-4-yl1-thiazol-2-yl} -amide)
Example 2: (S)-Pyrrolidine-1.2-dicarboxylic acid 2-amide 1 -((4-methyl-5- 2 -(2,2,2- trifluoro-1 J-dirhethyl-ethylVpyridin-4-yl -thia2ol-2-yll-amide
The title compound is prepared in analogy to the procedure described in Example 1 but with the following modifications. In Step 2.1 (corresponding to Step 1.1 of Example 1), the reaction mixture is stirred for 14 h at reflux. In Step 2.2 (corresponding to Step 1.2 of Example 1), the reaction mixture is stirred for 1 h at 85 °C and extracted with ethyl acetate after being quenched. In step 2.3 (corresponding to Step 1.3 of Example 1), the reaction mixture is stirred for 2.5 h at 120 °C. In Step 2.4 (corresponding to Step 1.4 of Example 1), the reaction mixture is stirred for 1 h at 83 °C and extracted with ethyl acetate after being quenched. In Step 2.5 (corresponding to Step 1.5 of Example 1), the reaction mixture is stirred for 1 h at 65 °C and trituration in methanol is not performed. In Step 2.6
(corresponding to Step 1.6 of Example 1), the crude product is not purified. In Step 2.7 (corresponding to Step 1.7 of Example 1), 3,3,3-trifluoro-2,2-dimethyl-propionyl chloride is used.
Title compound: ESI-MS: 442.0 [M+H]+; tR= 3.02 min (System 1); TLC: Rf = 0.35 (DCM/MeOH, 9: 1).
Example 3: Preparation of Polymorph Form A
(S)-Pyrrolidine-l,2-dicarboxylic acid 2-amide l-({4-methyl-5-[2-(2,2,2-trifluoro-l,l- dimethyl-ethyl)-pyridin-4-yl]-thiazol-2-yl}-amide) (10.0 g) was suspended in ethanol/water (85:15 v/v; 75 mL) and the mixture was heated to 75 °C. The solution was clear-filtered into a second flask and the first flask was then washed with ethanol/water (4:6 v/v; 20 mL), followed by water (10 mL). The clear solution was stirred at 75 °C for an additional 30 minutes. The clear solution was then cooled to 2 °C over 2 hours and the obtained thick suspension was stirred at 2 °C for an additional hour. The mixture was then filtered, and the flask and filter cake were washed with ethanol/water (1 :1 v/v; 20 mL), followed by ethyl acetate (10 mL). The wet filter cake was returned to the flask and suspended in ethyl acetate (75 mL). the mixture was heated to 78 °C and was stirred under reflux for 1 hour. During this time, 15 mL ethyl acetate was distilled off. The mixture was then cooled to 2 °C over 2 hours and the suspension was stirred at 2 °C for an additional hour. The mixture was filtered, and the flask and filter cake were washed with cold ethyl acetate (12 mL). The filter cake was then dried under 1-50 mbar vacuum at 50 °C to yield the polymorph form A (7.3 g).
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US9815898 | ANTIBODY MOLECULES TO PD-1 AND USES THEREOF |
2017-05-15
|
|
| US2017210733 | BENZOXAZEPIN OXAZOLIDINONE COMPOUNDS AND METHODS OF USE |
2017-04-07
|
|
| US2017210804 | ANTIBODY MOLECULES TO LAG-3 AND USES THEREOF |
2017-03-24
|
|
| US2017190777 | ANTIBODY MOLECULES TO TIM-3 AND USES THEREOF |
2017-03-17
|
|
| US2017166550 | BENZOTHIOPHENE-BASED SELECTIVE ESTROGEN RECEPTOR DOWNREGULATORS |
2016-12-09
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2016113932 | TREATMENT OF CANCERS USING PI3 KINASE ISOFORM MODULATORS |
2014-05-30
|
2016-04-28
|
| US2016067212 | USE OF INSULIN SIGNALING ANTAGONISTS, OPTIONALLY IN COMBINATION OF TRANSFECTION OF NON-BETA CELLS, FOR INDUCING INSULIN PRODUCTION |
2014-03-14
|
2016-03-10
|
| US2014186469 | PYRROLIDINE-1, 2-DICARBOXAMIDE DERIVATIVES |
2014-02-27
|
2014-07-03
|
| US9675595 | SYNERGISTIC COMBINATIONS OF PI3K- AND MEK-INHIBITORS |
2012-08-30
|
2014-06-26
|
| US2014235630 | COMPOSITIONS AND METHODS FOR THE TREATMENT OF PROLIFERATIVE DISEASES |
2012-09-28
|
2014-08-21
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2017136043 | THERAPEUTIC USES OF SELECTED PYRROLOPYRIMIDINE COMPOUNDS WITH ANTI-MER TYROSINE KINASE ACTIVITY |
2017-01-30
|
|
| US2017135960 | AGGREGATING MICROPARTICLES FOR MEDICAL THERAPY |
2016-11-11
|
|
| US9724352 | PYRROLO[2, 1-F[1, 2, 4]TRIAZINE COMPOUNDS, PREPARATION METHODS AND APPLICATIONS THEREOF |
2016-09-19
|
|
| US2017056336 | CO-TARGETING ANDROGEN RECEPTOR SPLICE VARIANTS AND MTOR SIGNALING PATHWAY FOR THE TREATMENT OF CASTRATION-RESISTANT PROSTATE CANCER |
2016-05-09
|
|
| US2017151264 | COMBINATION |
2015-05-21
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2016117439 | SUPERIOR BIOINFORMATICS PROCESS FOR IDENTIFYING AT RISK SUBJECT POPULATIONS |
2015-10-26
|
2016-04-28
|
| US2017096492 | DOSAGE AND ADMINISTRATION OF ANTI-IGF-1R, ANTI-ErbB3 BISPECIFIC ANTIBODIES, USES THEREOF AND METHODS OF TREATMENT THEREWITH |
2016-08-19
|
|
| US2017165246 | PHARMACEUTICAL COMBINATIONS COMPRISING A PI3K INHIBITOR FOR THE TREATMENT OF CANCER |
2015-02-10
|
|
| US9474754 | Pharmaceutical Combinations Comprising a B-RAF Inhibitor, and EGFR Inhibitor and Optionally a PI3K-Alpha Inhibitor |
2013-08-05
|
2015-09-24
|
| US2015111927 | PHARMACEUTICAL DIAGNOSTIC |
2013-03-27
|
2015-04-23
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US9776966 | Synthesis of 2-carboxamide cycloamino urea derivatives |
2017-04-06
|
2017-10-03
|
| US2016303137 | DUAL PI3K AND WNT PATHWAY INHIBITION AS A TREATMENT FOR CANCER |
2016-04-20
|
|
| US2016095842 | COMBINATION THERAPY CONTAINING A PI3K-ALPHA INHIBITOR AND FGFR KINASE INHIBITOR FOR TREATING CANCER |
2014-05-28
|
2016-04-07
|
| US2016030440 | ANTI-TUMORAL COMPOSITION COMPRISING A PI3KBETA-SELECTIVE INHIBITOR AND A PI3KALPHA-SELECTIVE INHIBITOR |
2014-04-03
|
2016-02-04
|
| US2016184311 | Combination Therapy for the Treatment of Cancer |
2014-08-07
|
2016-06-30
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2017166551 | BENZOTHIOPHENE-BASED SELECTIVE ESTROGEN RECEPTOR DOWNREGULATOR COMPOUNDS |
2016-12-09
|
|
| US9006270 | POLYMORPHS OF (S)-PYRROLIDINE-1, 2-DICARBOXYLIC ACID 2-AMIDE 1-(-AMIDE |
2012-06-19
|
2014-06-19
|
| US9650373 | Synthesis of 2-carboxamide cycloamino urea derivatives |
2012-03-01
|
2013-12-12
|
| US8980259 | COMBINATION THERAPY |
2013-03-13
|
2014-01-23
|
| US2016375033 | METHODS OF TREATMENT WITH TASELISIB |
2016-06-28
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2015291606 | MERTK-SPECIFIC PYRROLOPYRIMIDINE COMPOUNDS |
2015-04-03
|
2015-10-15
|
| US2015291609 | MERTK-SPECIFIC PYRIMIDINE COMPOUNDS |
2015-04-03
|
2015-10-15
|
| US9603850 | MERTK-SPECIFIC PYRAZOLOPYRIMIDINE COMPOUNDS |
2015-04-03
|
2015-10-15
|
| US2015259420 | ANTIBODY MOLECULES TO LAG-3 AND USES THEREOF |
2015-03-13
|
2015-09-17
|
| US9605070 | ANTIBODY MOLECULES TO TIM-3 AND USES THEREOF |
2015-01-30
|
2015-08-06
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2016117443 | BIOINFORMATICS PROCESS FOR IDENTIFYING AT RISK SUBJECT POPULATIONS |
2015-10-26
|
2016-04-28
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US9789203 | ANTIBODY DRUG CONJUGATES |
2014-03-12
|
2016-02-04
|
| US9683048 | ANTIBODY MOLECULES TO PD-1 AND USES THEREOF |
2015-01-23
|
2015-07-30
|
| US9227969 | COMPOUNDS AND COMPOSITIONS AS INHIBITORS OF MEK |
2014-08-05
|
2015-02-19
|
| US2014377258 | Treatment Of Cancers Using PI3 Kinase Isoform Modulators |
2014-05-30
|
2014-12-25
|
| US2015283142 | TREATMENT OF CANCERS USING PI3 KINASE ISOFORM MODULATORS |
2013-11-01
|
2015-10-08
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2016108123 | ANTIBODY MOLECULES TO PD-L1 AND USES THEREOF |
2015-10-13
|
2016-04-21
|
| US2017209574 | COMBINATION THERAPIES |
2015-10-02
|
|
| US2017224836 | ANTI-CDH6 ANTIBODY DRUG CONJUGATES |
2015-08-07
|
|
| US2017189409 | MEDICAL USE |
2015-05-21
|
|
| US2015320880 | ANTIBODY DRUG CONJUGATES |
2015-05-20
|
2015-11-12
|
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2017183348 | COMPOUNDS AND COMPOSITIONS AS INHIBITORS OF MEK |
2017-03-16
|
|
| US2017198041 | ANTIBODY MOLECULES TO TIM-3 AND USES THEREOF |
2017-02-14
|
|
| US2017121421 | ANTIBODY CONJUGATES COMPRISING TOLL-LIKE RECEPTOR AGONIST |
2016-10-25
|
|
| US9650393 | BENZOXAZEPIN OXAZOLIDINONE COMPOUNDS AND METHODS OF USE |
2016-07-01
|
|
| US9629836 | COMPOUNDS AND COMPOSITIONS AS INHIBITORS OF MEK |
2015-11-13
|
2016-05-19
|
/////////////////Alpelisib, CAS, 1217486-61-7, BYL-719, BYL719, UNII-08W5N2C97Q, BYL 719
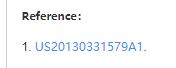
CC1=C(SC(=N1)NC(=O)N2CCCC2C(=O)N)C3=CC(=NC=C3)C(C)(C)C(F)(F)F