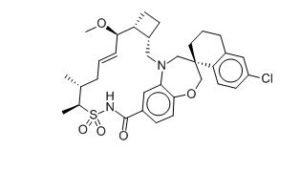
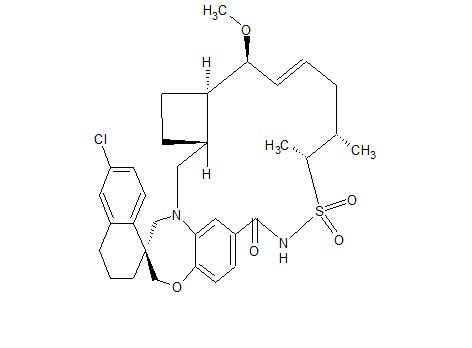
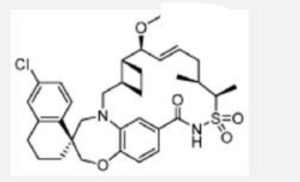
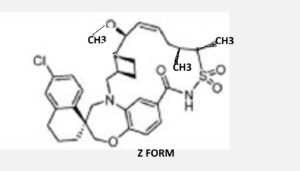
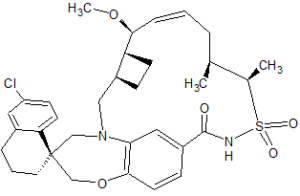AMG 176
PHASE 1, Amgen, Mcl-1 inhibitor, tumors
- Class Antineoplastics; Small molecules
- Mechanism of Action MCL1 protein inhibitors
- Phase I Multiple myeloma
- 01 Jun 2016 Phase-I clinical trials in Multiple myeloma (Second-line therapy or greater) in USA, Australia (IV) (NCT02675452)
- 12 Feb 2016 Amgen plans a first-in-human phase I trial for Multiple myeloma (Second-line therapy or greater) in USA, Germany and Australia (IV) (NCT02675452)
- 22 Dec 2015 Preclinical trials in Multiple myeloma in USA (IV) before December 2015

Synthesis
1 Kang catalyst used, ie Pyridine, 2,6-bis[(4R)-5,5-dibutyl-4,5-dihydro-4-phenyl-2-oxazolyl]-
2 Martin’s reagent to get CHO group
3 Hydrolysis or Hydrogenolysis of Carboxylic Esters :p-MeC6H4SO3H
4 R:(Me3Si)2NH •Li,
5 Hydrolysis of Acetals CF3SO3H
6 Fe, AcOH CYCLIZATION
7 l-Camphor-SO3H, Na+ •(AcO)3BH-,
8 SOCl2, MeOH ESTERIFICATION
9 OXIDATION
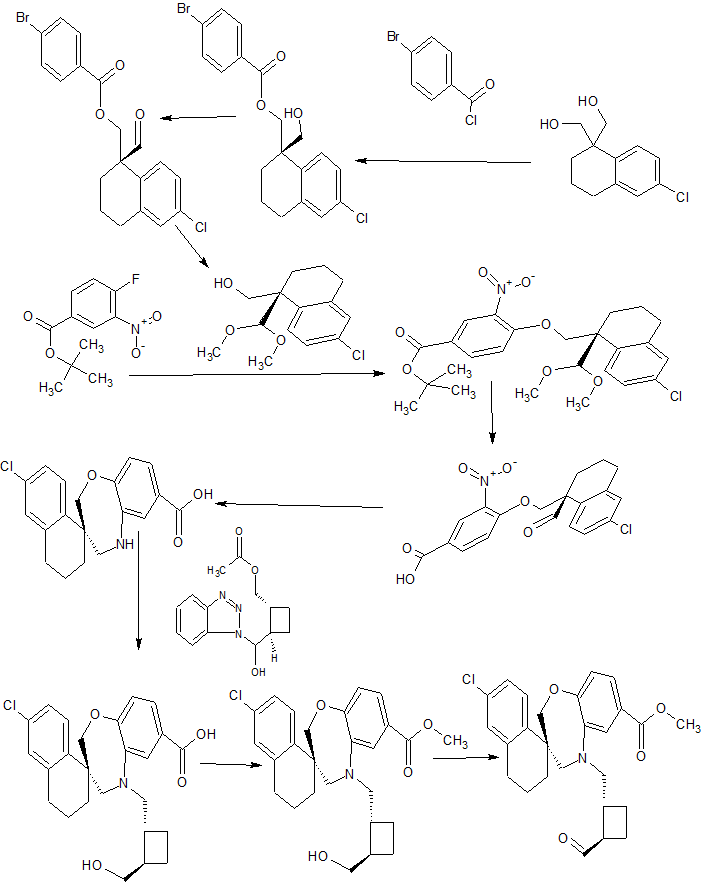
CONTD………..
10 GRIGNARD BuLi, Me(CH2)4Me,
11 Hydrogenolysis of Carboxylic Esters
12 Acylation INVOLVING NITROGEN ATOM
13 CYCLIZATION , Ruthenium, [1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(phenylmethylene)(tricyclohexylphosphine)-, (SP-5-41)-
14 METHYL IODIDE, Alkylation TO GET AMD 176
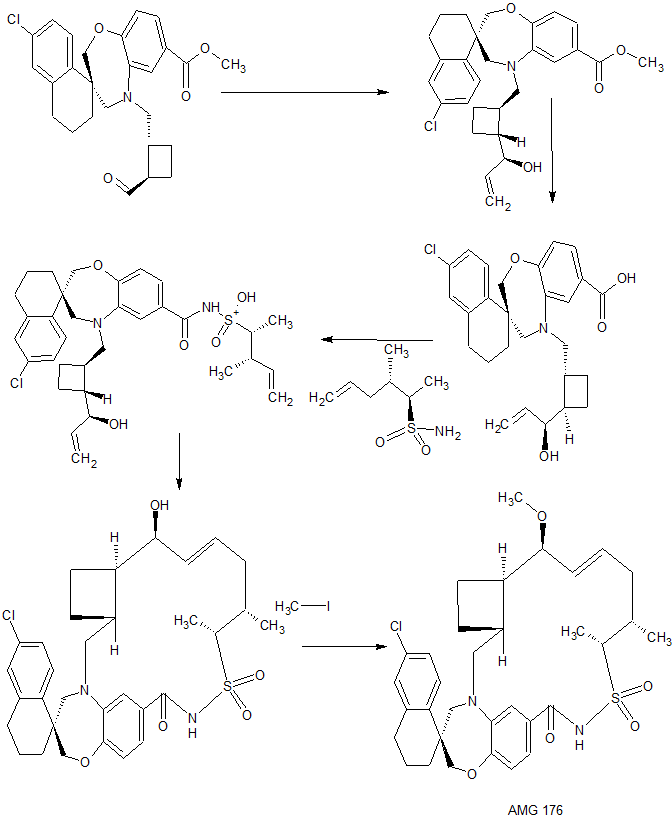
AMG 176
One common characteristic of human cancer is overexpression of Mcl-1. Mcl-loverexpression prevents cancer cells from undergoing programmed cell death (apoptosis), allowing the cells to survive despite widespread genetic damage.
Mcl-1 is a member of the Bcl-2 family of proteins. The Bcl-2 family includes pro-apoptotic members (such as BAX and BAK) which, upon activation, form a homo-oligomer in the outer mitochondrial membrane that leads to pore formation and the escape of mitochondrial contents, a step in triggering apoptosis. Antiapoptotic members of the Bcl-2 family (such as Bcl-2, Bcl-XL, and Mcl-1) block the activity of BAX and BAK. Other proteins (such as BID, BIM, BIK, and BAD) exhibit additional regulatory functions.
Research has shown that Mcl- 1 inhibitors can be useful for the treatment of cancers. MCl-1 is overexpressed in numerous cancers. See Beroukhim et al. (2010) Nature 463, 899-90. Cancer cells containing amplifications surrounding the Mcl-1 and Bcl-2-1-1 anti-apoptotic genes depend on the expression of these genes for survival. Beroukhim et al. Mcl- 1 is a relevant target for the re-iniation of apoptosis in numerous cancer cells. See G. Lessene, P. Czabotar and P.
Colman, Nat. Rev. Drug. Discov., 2008, 7, 989-1000; C. Akgul Cell. Mol. Life
Sci. Vol. 66, 2009; and Arthur M. Mandelin II, Richard M. Pope, Expert Opin. Ther. Targets (2007) l l(3):363-373.
New compositions and methods for preparing and formulating Mcl-1 inhibitors would be useful.
PATENT
WO 2016033486
https://www.google.com/patents/WO2016033486A1?cl=ru
GENERAL SYNTHETIC SCHEMES
General Procedure 1

Intermediates III can be prepared using standard chemistry techniques. For example, cyclobutane carbaldehyde II was combined with oxazepine I in an appropriate solvent at a temperature below RT, preferably about 0°C. Sodium cyanoborohydride was added, and the mixture was added to NaOH solution, to provide compound III.
General Procedure 2

Intermediate AA Intermediate EE IV
Intermediates IV can be prepared using standard peptide like chemistry. For example, DMAP was added to carboxylic acid Intermediate AA and Intermediate EE in an appropriate solvent at a temperature below RT, preferably about 0°C, followed by the addition of EDC hydrochloride. The mixture was warmed to ambient temperature, to provide carboxamide IV.
General Procedure 3

EXAMPLE A
Example A intermediates can be prepared using standard chemistry techniques. For example, carboxamide IV was combined with DCM followed by the addition of Hoveyda-Grubbs II. The mixture was cooled to ambient temperature to provide Example A.
General Procedure 4

Intermediate AA Intermediate EE
Intermediates V can be prepared using standard chemistry techniques. For example, Intermediate AA was combined with Intermediate EE in an appropriate solvent followed by the addition of Hoveyda-Grubbs II to provide compound V.
General Procedure 5

Example A intermediates can be prepared using standard chemistry techniques. For example, N,N-dimethylpyridin-4-amine was combined with compound VI in an appropriate solvent at a temperature below RT, preferably about 0°C, followed by the addition of N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride. The resulting mixture warmed to ambient temperature to provide Example A.
General Procedure 6

Example B intermediates can be prepared using standard chemistry techniques. For example, sodium hydride was added to a solution of Example A at a temperature below RT, preferably about 0°C, followed by the addition of Mel. The resulting mixture warmed to ambient temperature to provide Example B.
General Pr

Intermediates such as Example C can be prepared using standard chemistry techniques. For example, Example A and/or B and/or VII and platinum (IV) oxide were combined in an appropriate solvent at ambient temperature to provide Example C.
Compounds of the present invention generally can be prepared combining and further elaborating synthetic intermediates generated from commercially available starting materials. The syntheses of these intermediates are outlined below and further exemplification is found in the specific examples provided.
EXAMPLE 4. (1S,3’R,6’R,7’S,8’E,1 l’S,12’R)-6-CHLORO-7′-METHOXY-11′, 12 ‘-DIMETHYL-3 ,4-DIHYDRO-2H, 15 ‘H-SPIRO [NAPHTHALENE- 1 ,22’-[20]OXA[13]THIA[1,14]DIAZATETRACYCLO[14.7.2.03‘6.019‘24]PENTACOS A[8, 16, 18,24]TETRAEN]-15′-ONE- 13 ‘, 13 ‘-DIOXIDE

To a slurry of (1 S,3’R,6’R,7’S,8’E, 1 l’S, 12’R)-6-chloro-7′-hydroxy-l l’,12′-dimethyl-3,4-dihydro-2h, 15’h-spiro[naphthalene-l,22′- [20]oxa[13]thia[l, 14]diazatetracyclo[14.7.2.03‘6.019‘24]pentacosa[8,16, 18,24]tetra en]-15′-one 13 ‘, 13 ‘-dioxide (Example 2; 32.6 g, 49.1 mmol) (containing 9.8% toluene, starting material was not completely soluble in Me-THF) and Mel (15.2 mL, 245 mmol) in Me-THF (820 mL) was added KHMDS (1.0 M in THF, 167 mL, 167 mmol) dropwise for 30 min while maintaining reaction temperature between – 44°C and – 38°C under N2. After the mixture was stirred at – 44°C for 30 min, the reaction was allowed to warm to rt and stirred for 1.5 h (LC/MS confirmed the reaction was complete). The reaction mixture was cooled to 5°C, quenched (170 mL of sat. aqueous NH4C1 and 170 mL of FLO) while maintaining temperature between 5°C and 14°C, and acidified (340 mL of 10% aqueous citric acid). The organic layer was separated and the aqueous layer was back-extracted with EtOAc (500 mL). The combined organic layers were washed with brine (3 x 500 mL), dried (MgS04), and concentrated under reduced pressure to provide a crude target compound (30.1 g, 49.1 mmol, quantitatively) (purity >98% with no over 1% major impurity from HPLC) as a bright yellow solid. After the same scale reaction was repeated four times, all the crude products (4 x 49.1 mmol = 196 mmol) were dissolved in EtOAc, combined, and concentrated under reduced pressure. Then the combined crude product was recrystallized as follows:
ethanol (800 mL) was added to the crude product and the resulting slurry solution was shaken while heating the solution for 20 min. H20 (250 mL) was added dropwise for 30 min at rt and the slurry was cooled down to 0°C. After the slurry was kept in an ice bath for 4 h, the solid product was filtered through filter paper. The filter cake was rinsed with ice-cold 30% FLO in EtOH (300 mL) and air-dried for 2 days. The product was further dried under high vacuum at 40°C for 4 days to provide the pure target compound (1 15 g, 188 mmol, 96 % yield) as a
white solid. XH NMR (600 MHz, DMSO-i¾) δ 11.91 (s, 1 H), 7.65 (d, J= 8.6 Hz, 1 H), 7.27 (dd, J= 8.5, 2.3 Hz, 1 H), 7.17 (d, J= 2.4 Hz, 1 H), 7.04 (dd, J= 8.2, 2.0 Hz, 1 H), 6.90 (d, J= 8.2 Hz, 1 H), 6.76 (d, J= 1.8 Hz, 1 H), 5.71 (ddd, J= 15.1, 9.7, 3.5 Hz, 1 H), 5.50 (ddd, J= 15.2, 9.2, 1.1 Hz, 1 H), 4.08 (qd, J= 7.2, 7.2, 7.2, 1.5 Hz, 1 H), 4.04 (d, J= 12.3 Hz, 1 H), 3.99 (d, J= 12.3 Hz, 1 H), 3.73 (d, J= 14.9 Hz, 1 H), 3.56 (d, J= 14.1 Hz, 1 H), 3.53 (dd, J= 9.1, 3.3 Hz, 1 H), 3.19 (d, J= 14.1 Hz, 1 H), 3.09 (s, 3 H), 3.03 (dd, J= 15.4, 10.4 Hz, 1 H), 2.79 (dt, J= 17.0, 3.5, 3.5 Hz, 1 H), 2.69 (ddd, J= 17.0, 10.7, 6.3 Hz, 1 H), 2.44-2.36 (m, 1 H), 2.24-2.12 (m, 2 H), 2.09 (ddd, J= 15.5, 9.6, 2.3 Hz, 1 H), 1.97 (dt, J = 13.6, 3.6, 3.6 Hz, 1 H), 1.91-1.80 (m, 4 H), 1.80-1.66 (m, 3 H), 1.38 (td, J= 12.3, 12.3, 3.5 Hz, 1 H), 1.33 (d, J= 7.2 Hz, 3 H), 0.95 (d, J= 6.8 Hz, 3 H); [cc]D (24°C, c = 0.0103 g/mL, DCM) = – 86.07 °; m.p. 222.6 – 226.0°C; FT-IR (KBr): 3230 (b), 2931 (b), 1688 (s), 1598 (s), 1570 (s), 1505 (s), 1435 (s), 1384 (s), 1335 (s), 1307 (s), 1259 (s), 1155 (s), 1113 (s), 877 (s), 736 (s) cm“1; Anal. Calcd. for C33H41CIN2O5S: C, 64.64; H, 6.74; N, 4.57; CI, 5.78; S, 5.23. Found: C, 64.71; H, 6.81; N, 4.65; CI, 5.81; S, 5.11; HRMS (ESI) m/z 613.2493 [M + H]+ (C33H41CIN2O5S requires 613.2503).
The mother liquor was concentrated under reduced pressure and further purification of the residue by flash column chromatography (200 g S1O2, 10% and 10% to 45% and 45% EtO A/Hex w/ 0.3% AcOH, gradient elution) provided additional pure product (3.1 g, 5.1 mmol, 2.6%) as an off-white solid.
EXAMPLE 5. (1S,3’R,6’R,7’S,8’Z,1 l’S,12’R)-6-CHLORO-7′-METHOXY-11 ‘, 12 ‘-DIMETHYL-3 ,4-DIHYDRO-2H, 15 Ή-SPIRO [NAPHTHALENE- 1 ,22’-[20]OXA[13]THIA[1,14]DIAZATETRACYCLO[14.7.2.03‘6.019‘24]PENTACOS A[8, 16, 18,24]TETRAEN]- 15′-ONE 13 ‘, 13’-DIOXIDE

To a solution of (1S,3’R,6’R,7’S,8’Z,1 l’S,12’R)-6-chloro-7′-hydroxy-i r,12′-dimethyl-3,4-dihydro-2h,15’h-spiro[naphthalene-l,22′-[20]oxa[13]thia[l,14]diazatetracyclo[14.7.2.03‘6.019‘24]pentacosa[8,16,18,24]tetra en]-15′-one 13 ‘,13 ‘-dioxide (Example 3; 34 mg; 0.057 mmol) in THF cooled to 0°C was added sodium hydride (60% dispersion in mineral oil; 22.70 mg, 0.567 mmol). The reaction mixture was stirred at 0 °C for 20 min, and then Mel (0.018 mL, 0.284 mmol) was added. The reaction mixture was stirred at ambient temperature for 1 h, then quenched with aqueous NH4CI, and diluted with
EtOAc. The organic layer was dried over MgS04 and concentrated. Purification of the crude material via column chromatography eluting with 10-40 % EtOAc (containing 0.3% AcOH)/heptanes provided (lS,3’R,6’R,7’S,8’Z,l l’S,12’R)-6-chloro-7′-methoxy-l l’,12′-dimethyl-3,4-dihydro-2h,15’h-spiro[naphthalene-l,22′-[20]oxa[13]thia[l,14]diazatetracyclo[14.7.2.03‘6.019‘24]pentacosa[8,16,18,24]tetra en]-15′-one 13 ‘,13 ‘-dioxide (34 mg, 0.054 mmol, 95% yield). ¾ NMR (400MHz, CD2C12) δ 8.29 (s, 1H), 7.71 (d, J=8.4 Hz, 1H), 7.17 (dd, J=2.2, 8.5 Hz, 1H), 7.09 (d, J=2.3 Hz, 1H), 7.01 (dd, J=1.6, 7.8 Hz, 1H), 6.92 (d, J=8.2 Hz, 1H), 6.88 (s, 1H), 5.90 – 5.80 (m, 1H), 5.54 (t, J=10.2 Hz, 1H), 4.14 – 4.04 (m, 3H), 3.87 – 3.79 (m, 2H), 3.73 (d, J=14.7 Hz, 1H), 3.32 (d, J=14.5 Hz, 1H), 3.23 (s, 3H), 3.28 -3.19 (m, 1H), 2.82 – 2.73 (m, 2H), 2.62 (t, J=10.6 Hz, 1H), 2.55 – 2.44 (m, 1H), 2.29 – 2.21 (m, 1H), 2.10 – 1.97 (m, 4H), 1.97 – 1.80 (m, 4H), 1.75 (dd, J=8.9, 18.7 Hz, 1H), 1.48 (d, J=7.4 Hz, 3H), 1.43 (br. s., 1H), 1.08 (d, J=6.5 Hz, 3H). MS (ESI, +ve ion) m/z 613.3 (M+H)+.
//////////////AMG 176, PHASE 1, Amgen, Mcl-1 inhibitor, tumors
Last talk in AM #MEDI 1st time disclosures is from Sean Brown of @Amgen on an Mcl-1 inhibitor to treat tumors #ACSSanFran
Clc5cc6CCC[C@@]4(CN2C[C@H]1CC[C@H]1[C@H](OC)C=CC[C@@H](C)[C@H](C)S(=O)(=O)NC(=O)c3cc2c(cc3)OC4)c6cc5