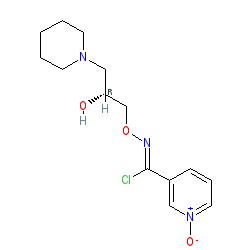
ARIMOCLOMOL
アリモクロモル;
| Formula |
C14H20ClN3O3
|
|---|---|
| Exact mass |
313.1193
|
| Mol weight |
313.7799
|
CAS 289893-25-0
289893-26-1 (Arimoclomol maleate);
INN 8300
N-[(2R)-2-hydroxy-3-piperidin-1-ylpropoxy]-1-oxidopyridin-1-ium-3-carboximidoyl chloride
BRX 220
Arimoclomol maleate is in a phase III clinical trials by Orphazyme for the treatment of Niemann-Pick disease type C (NP-C). It is also in phase II clinical studies for the treatment of amyotrophic lateral sclerosis (ALS).
Arimoclomol (INN; originally codenamed BRX-345, which is a citrate salt formulation of BRX-220) is an experimental drug developed by CytRx Corporation, a biopharmaceutical company based in Los Angeles, California. In 2011 the worldwide rights to arimoclomol were bought by Danish biotech company Orphazyme ApS.[1] The European Medicines Agency (EMA) and U.S. Food & Drug Administration (FDA) granted orphan drug designation to arimoclomol as a potential treatment for Niemann-Pick type C in 2014 and 2015 respectively.[2][3]
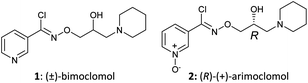 |
||
| Fig. 1 Structures of (±)-bimoclomol (1) and (R)-(+)-arimoclomol (2). | ||
1. WO0179174A1.
PATENT
WO/2022/106614PROCESSES FOR PREPARING ARIMOCLOMOL CITRATE AND INTERMEDIATES THEREOF
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022106614&_cid=P22-L3S8T3-04206-1
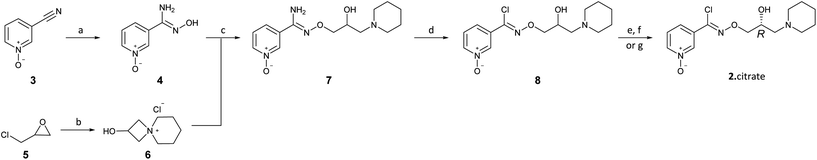
The present disclosure provides an optimized four-step process for preparing an ultra-pure composition comprising arimoclomol citrate, i.e. N-{[(2R)-2-hydroxy-3-piperidin-l-ylpropyl]oxy}pyridine-3-carboximidoyl chloride 1-oxide citrate. The optimized process comprises a plurality of optimized sub-steps, each contributing to an overall improved process, providing the ultra-pure composition comprising arimoclomol citrate. The ultra-pure composition comprising arimoclomol citrate meets the medicines agencies’ high regulatory requirements. An overview of the four-steps process is outlined below:
Step 1: Overview of process for preparing ORZY-01
Step 2: Overview of process for preparing ORZY-03
Step 4: Overview of process for preparing BRX-345 (ORZY-05)
The previously reported two-step synthesis of ORZY-01 as shown below includes a 2 hour reflux in step 1A, followed by purification of intermediate compound (V) to increase the batch quality.
PAPER
https://pubs.rsc.org/en/content/articlehtml/2017/ob/c7ob02578e
DOI: 10.1039/C7OB02578E (Communication) Org. Biomol. Chem., 2017, 15, 9794-9799
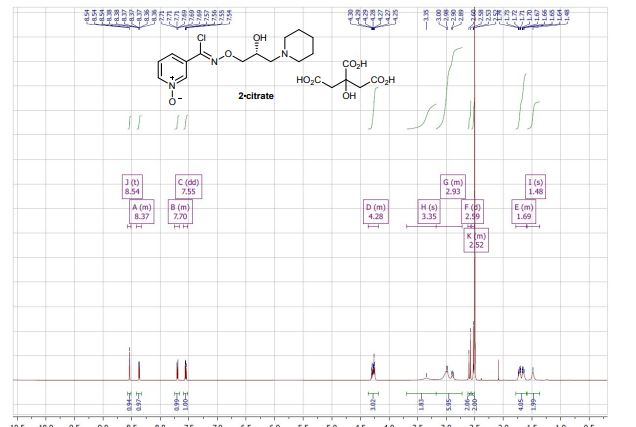
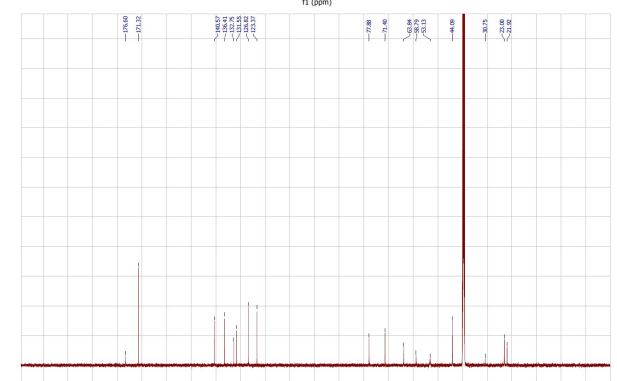
- (R,Z)-3-(N′-(2-Hydroxy-3-(piperidine-1-yl)propoxy)carboximi-oylchloride)pyridine-1-oxide citrate (2-citrate, arimoclomol citrate) was prepared as an off-white amorphous solid (164 mg): m.p. 161–162 °C; [α]20D +8.0° (c = 1, H2O); IR νmax (neat): 3423, 3228, 2949, 2868, 1722, 1589, 1483, 1433, 1307, 1128, 972, 829 cm−1; 1H NMR (600 MHz, d6-DMSO) δ: 8.54 (t, J = 1.5 Hz, 1H), 8.39–8.35 (m, 1H), 7.72–7.68 (m, 1H), 7.55 (dd, J = 8.0, 6.5 Hz, 1H), 4.28 (ddd, J = 17.6, 13.3, 7.4 Hz, 3H), 3.35 (br. s, 2H), 3.13–2.74 (m, 6H), 2.59 (d, J = 15.2 Hz, 2H), 2.56–2.51 (m, 2H), 1.77–1.61 (m, 4H), 1.48 (s, 2H); 13C NMR (151 MHz, d6-DMSO) δ: 176.6, 171.3, 140.5, 136.4, 132.7, 131.5, 126.8, 123.3, 77.8, 71.4, 63.8, 58.7, 53.1, 44.0, 30.7, 23.0, 21.9; HRMS (m/z TOF MS ES+) for C14H20ClN3O3 [M + H]+ calc. 314.1271, observed 314.1263; SFC er purity R
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, >99
S, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1. - (R,Z)-3-(N′-(2-Hydroxy-3-(piperidine-1-yl)propoxy)carboximi-oylchloride)pyridine maleate ((R)-1-maleate, bimoclomol maleate) was prepared as an off-white amorphous solid (70 mg): m.p. 137–138 °C; [α]20D +6.0° (c = 1, MeOH); IR νmax (neat): 3269, 2937, 1577, 1477, 1440, 1348, 1205, 1070, 981, 864 cm−1; 1H NMR (600 MHz, d6-DMSO) δ: 9.09 (s, 1H), 9.01–8.98 (m, 1H), 8.73 (dd, J = 4.8, 1.5 Hz, 1H), 8.24–8.06 (m, 1H), 7.57 (ddd, J = 8.1, 4.8, 0.6 Hz, 1H), 6.02 (d, J = 4.0 Hz, 2H), 5.93 (s, 1H), 4.40–4.21 (m, 3H), 3.60–3.28 (m, 3H), 3.20 (d, J = 11.8 Hz, 1H), 3.12–3.05 (m, 1H), 3.03–2.83 (m, 2H), 1.84–1.55 (m, 5H), 1.38 (s, 1H); 13C NMR (151 MHz, d6-DMSO) δ: 167.1, 151.7, 147.4, 136.0, 135.1, 134.6, 127.9, 123.9, 77.2, 63.1, 58.0, 54.1, 51.1, 22.2, 21.3; HRMS (m/z TOF MS ES+) for C14H20ClN3O2 [M + H]+ calc. 298.1322, observed 298.1319; SFC er purity R
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, 98
S, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
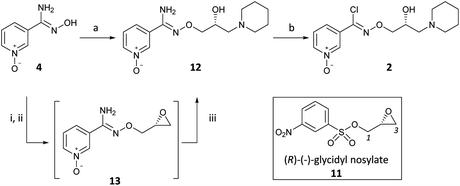
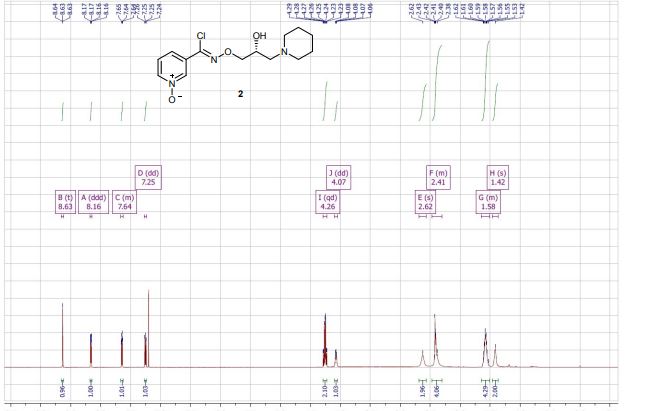
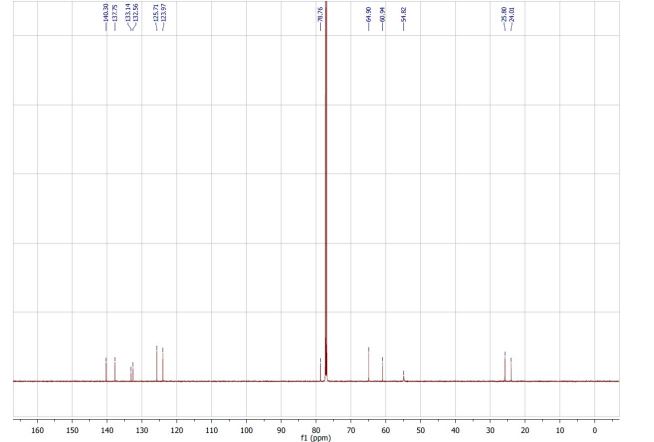
(R,Z)-3-(N’-(2-hydroxy-3-(piperidin-1-yl)propoxy)carboximidoyl chloride)pyridine-1-oxide1 – (R)-(+)-Arimoclomol – 2 A solution of (R,Z)-3-(N’-(2-hydroxy-3-(piperidin-1-yl)propoxy)carbamimidoyl)pyridine-1-oxide 12 (205 mg, 0.70 mmol) in conc. hydrochloric acid (1.1 mL, 13.9 mmol) and water (3 mL) was cooled to -5 °C for 15 minutes. Sodium nitrite (63 mg, 0.91 mmol) in water (0.5 mL) was then added dropwise to the reaction mixture and the reaction was stirred at -5 °C for 2.5 hours. The reaction mixture was made alkaline with NaOH (7 M, 3 mL). An additional 10 mL of water was added followed by DCM (30 mL) containing EtOAc (5 mL) and the organics were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by FCC on Biotage Isolera using Biotage SNAP 10 g Si cartridge eluting with gradient elution 0-30% MeOH:DCM both containing 0.1% Et3N to afford the title compound (160 mg, 73% yield) as a colourless semi-solid. Analytical data was consistent with literature values. See ESI section SFC traces for specific enantiomeric ratios of 2 synthesised under the various methodologies quoted in the text. Optical rotation was not determined as it was determined in the ultimate product of this 2·citrate and comparative run times on SFC. 1H NMR (600 MHz, CDCl3) δ: 8.63 (t, J = 1.4 Hz, 1H), 8.16 (ddd, J = 6.4, 1.6, 0.9 Hz, 1H), 7.66 – 7.62 (m, 1H), 7.25 (dd, J = 8.0, 6.6 Hz, 1H), 4.26 (qd, J = 11.3, 5.2 Hz, 2H), 4.07 (dd, J = 9.2, 4.7 Hz, 1H), 2.62 (s, 2H), 2.47 – 2.31 (m, 4H), 1.65 – 1.51 (m, 4H), 1.42 (s, 2H); 13C NMR (151 MHz, CDCl3) δ: 140.3, 137.7, 133.1, 132.5, 125.7, 123.9, 78.7, 64.9, 60.9, 54.8, 25.8, 24.0.
(R,Z)-3-(N’-(2-hydroxy-3-(piperidin-1-yl)propoxy)carboximidoyl chloride)pyridine-1-oxide citrate
(R)-(+)- Arimoclomol citrate – 2·citrate (R,Z)-3-(N’-(2-hydroxy-3-(piperidin-1-yl)propoxy)carboximidoyl chloride)pyridine-1-oxide (159 mg, 0.51 mmol) was dissolved in acetone (3 mL) and citric acid (97 mg, 0.51 mmol) was added. The reaction mixture was left to stir at room temperature for 18 hours. After this time the mixture was sonicated and the precipitate was filtered, rinsed with cold acetone (1 mL) and dried under vacuum to afford the title compound (165 mg, 64% yield) as a white amorphous solid. Analytical data was consistent with literature values. m.p. 161-162 °C, Acetone (lit. 163-165 °C, EtOH); [α]D 20 +8.0 (c=1, H2O); IR νmax (neat): 3423, 3228, 2949, 2868, 1722, 1589, 1483, 1433, 1307, 1128, 972, 829 cm-1; 1H NMR (600 MHz, d6-DMSO) δ: 8.54 (t, J = 1.5 Hz, 1H), 8.39 – 8.35 (m, 1H), 7.72 – 7.68 (m, 1H), 7.55 (dd, J = 8.0, 6.5 Hz, 1H), 4.28 (ddd, J = 17.6, 13.3, 7.4 Hz, 3H), 3.35 (br. s, 2H), 3.13 – 2.74 (m, 6H), 2.59 (d, J = 15.2 Hz, 2H), 2.56 – 2.51 (m, 2H), 1.77 – 1.61 (m, 4H), 1.48 (s, 2H); 13C NMR (151 MHz, d6-DMSO) δ: 176.6, 171.3, 140.5, 136.4, 132.7, 131.5, 126.8, 123.3, 77.8, 71.4, 63.8, 58.7, 53.1, 44.0, 30.7, 23.0, 21.9; HRMS (m/z TOF MS ES+) for C14H20ClN3O3 [M+H]+ calc. 314.1271, observed 314.1263; SFC er purity R:S >99:1
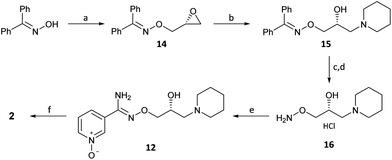
Procedure for the conversion of (R)-(+)-Bimoclomol 1 into (R)-(+)-Arimoclomol 2 To a solution of (R)-(+)-bimoclomol (61 mg, 0.21 mmol) in acetone (2 mL) was added benzenesulfonic acid (33 mg, 0.21 mmol). The reaction mixture was stirred at room temperature for 1.5 hours. The reaction mixture was concentrated in vacuo. Separately to a suspension of hydrogen peroxide-urea adduct (39 mg, 0.41 mmol) in acetonitrile (6 mL) at -5°C (ice-salt bath) was added trifluoroacetic anhydride (58 μL, 0.41 mmol) dropwise. A suspension of (R)-(+)-bimoclomol, 1, benzenesulfonic acid salt, as made above, in acetonitrile (3 mL) was then added dropwise to this solution. The reaction mixture was stirred for 18 hours, whilst slowly warming to room temperature. Aqueous Na2S2O5 solution (0.5 M, 1 mL) was added and the reaction mixture stirred for 1 hour. The reaction mixture was made alkaline with NaOH (7 M) and extracted with DCM (2 x 30 mL). The combined organics were washed with brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by FCC on a Biotage Isolera using Biotage SNAP 10g Si cartridge eluting with gradient elution 0-35% MeOH in DCM to afford the title compound (35 mg, 55% yield) as a colourless semi-solid. Analytical data of the products was consistent with literature and/or previous samples synthesised above.
https://www.rsc.org/suppdata/c7/ob/c7ob02578e/c7ob02578e1.pdf
//////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Mechanism of action
Arimoclomol is believed to function by stimulating a normal cellular protein repair pathway through the activation of molecular chaperones. Since damaged proteins, called aggregates, are thought to play a role in many diseases, CytRx believes that arimoclomol could treat a broad range of diseases.
Arimoclomol activates the heat shock response.[4][5][6][7][8][9] It is believed to act at Hsp70.[10]
History
Arimoclomol has been shown to extend life in an animal model of ALS[11] and was well tolerated in healthy human volunteers in a Phase I study. CytRx is currently conducting a Phase II clinical trial.[12]
Arimoclomol also has been shown to be an effective treatment in an animal model of Spinal Bulbar Muscular Atrophy (SBMA, also known as Kennedy’s Disease).[13]
Arimoclomol was discovered by Hungarian researchers, as a drug candidate to treat insulin resistance[14][15] and diabetic complications such as retinopathy, neuropathy and nephropathy. Later, the compound, along with other small molecules, was screened for further development by Hungarian firm Biorex, which was sold to CytRx Corporation, who developed it toward a different direction from 2003.
References
- ^ “CytRx Sells Molecular Chaperone Assets to Orphazyme in Deal Worth $120M | GEN Genetic Engineering & Biotechnology News – Biotech from Bench to Business | GEN”. GEN. 17 May 2011.
- ^ “European Medicines Agency – – EU/3/14/1376”. www.ema.europa.eu. Archived from the original on 2017-07-28. Retrieved 2022-02-15.
- ^ “Search Orphan Drug Designations and Approvals”. www.accessdata.fda.gov.
- ^ Kalmar B, Greensmith L (2009). “Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects”. Cell. Mol. Biol. Lett. 14 (2): 319–35. doi:10.2478/s11658-009-0002-8. PMC 6275696. PMID 19183864.
- ^ Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L (April 2004). “Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice”. Nat. Med. 10 (4): 402–5. doi:10.1038/nm1021. PMID 15034571. S2CID 2311751.
- ^ Kalmar B, Greensmith L, Malcangio M, McMahon SB, Csermely P, Burnstock G (December 2003). “The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury”. Exp. Neurol. 184 (2): 636–47. doi:10.1016/S0014-4886(03)00343-1. PMID 14769355. S2CID 5316222.
- ^ Rakonczay Z, Iványi B, Varga I, et al. (June 2002). “Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats”. Free Radic. Biol. Med. 32 (12): 1283–92. doi:10.1016/S0891-5849(02)00833-X. PMID 12057766.
- ^ Kalmar B, Burnstock G, Vrbová G, Urbanics R, Csermely P, Greensmith L (July 2002). “Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats”. Exp. Neurol. 176 (1): 87–97. doi:10.1006/exnr.2002.7945. PMID 12093085. S2CID 16071543.
- ^ Benn SC, Brown RH (April 2004). “Putting the heat on ALS”. Nat. Med. 10 (4): 345–7. doi:10.1038/nm0404-345. PMID 15057226. S2CID 11434434.
- ^ Brown IR (October 2007). “Heat shock proteins and protection of the nervous system”. Ann. N. Y. Acad. Sci. 1113 (1): 147–58. Bibcode:2007NYASA1113..147B. doi:10.1196/annals.1391.032. PMID 17656567. S2CID 36782230.
- ^ Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, Greensmith L (October 2008). “Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS”. J. Neurochem. 107 (2): 339–50. doi:10.1111/j.1471-4159.2008.05595.x. PMID 18673445.
- ^ “Phase II/III Randomized, Placebo-Controlled Trial of Arimoclomol in SOD1 Positive Familial Amyotrophic Lateral Sclerosis – Full Text View – ClinicalTrials.gov”. Archived from the original on 11 May 2009. Retrieved 2009-05-18.
- ^ Malik B, Nirmalananthan N, Gray A, La Spada A, Hanna M, Greensmith L (2013). “Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: implications for therapy”. Brain. 136 (3): 926–943. doi:10.1093/brain/aws343. PMC 3624668. PMID 23393146.
- ^ Kürthy M, Mogyorósi T, Nagy K, et al. (June 2002). “Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models”. Ann. N. Y. Acad. Sci. 967 (1): 482–9. Bibcode:2002NYASA.967..482K. doi:10.1111/j.1749-6632.2002.tb04306.x. PMID 12079878. S2CID 19585837.
- ^ Seböková E, Kürthy M, Mogyorosi T, et al. (June 2002). “Comparison of the extrapancreatic action of BRX-220 and pioglitazone in the high-fat diet-induced insulin resistance”. Ann. N. Y. Acad. Sci. 967 (1): 424–30. Bibcode:2002NYASA.967..424S. doi:10.1111/j.1749-6632.2002.tb04298.x. PMID 12079870. S2CID 23338560.
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H20ClN3O3 |
| Molar mass | 313.78 g·mol−1 |
| 3D model (JSmol) | |
| |
|
/////////ARIMOCLOMOL, アリモクロモル , BRX 220, INN 8300, Arimoclomol maleate, phase III, clinical, Orphazyme , Niemann-Pick disease type C, phase II, amyotrophic lateral sclerosis, (ALS)