AUR 101
AUR101-201
ANTIINNFLAMATORY
AUR-101, a ROR gamma inverse agonist for autoimmune disorders like psoriasis
AUR-101 is an ROR-gammaT inverse agonist in phase II clinical development at Aurigene for the treatment of patients with moderate-to-severe chronic plaque-type psoriasis.
- DrugsAUR 101 (Primary)
- IndicationsPlaque psoriasis
- FocusAdverse reactions; First in man
- AcronymsINDUS
- SponsorsAurigene Discovery Technologies
- OriginatorAurigene Discovery Technologies
- ClassAntipsoriatics; Small molecules
- Mechanism of ActionNuclear receptor subfamily 1 group F member 3 inverse agonists
- Phase IIPsoriasis
- 28 Aug 2021No recent reports of development identified for phase-I development in Psoriasis(In volunteers) in Australia (PO, Tablet)
- 23 Apr 2021Aurigene Discovery Technologies plans a phase II INDUS-3 trial for Psoriasis in USA (PO) in May 2021 (NCT04855721)
- 15 Apr 2021Aurigene Discovery Technologies completes a phase II trial in Psoriasis in India (PO) (NCT04207801)
- CDSCO
- https://www.cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadCTApprovals/Aurigene20.pdf
- NCT04207801
AURIGENE ANNOUNCES FIRST PATIENT DOSED WITH AUR101 IN PHASE II STUDY IN PATIENTS WITH MODERATE TO SEVERE PSORIASIS
https://www.aurigene.com/aurigene-announces-first-patient-dosed-with-aur101-in-phase-ii-study-in-patients-with-moderate-to-severe-psoriasis/
PRESS RELEASE
Aurigene Announces First Patient Dosed with AUR101 in Phase II Study in Patients with Moderate to Severe Psoriasis
Bangalore, February 17, 2020 — Aurigene, a development stage biotechnology company, today announced dose administration for the first patient in INDUS-2, a Phase II double blind placebo-controlled three-arm study of AUR101 in patients with moderate to severe psoriasis. AUR101 is an oral small molecule inverse agonist of RORγ and has shown desirable pharmacodynamic modulation of IL-17 and acceptable safety in a completed Phase I human study conducted in Australia.
“The initiation of this Phase II study under a US FDA IND represents a significant milestone for Aurigene, as it marks the first program which Aurigene has led from the bench side to the clinic all by itself,” said Murali Ramachandra, PhD, Chief Executive Officer of Aurigene. “We look forward to producing important clinical data by the end of 2020 to guide our future development plans and demonstrating Aurigene’s unique expertise in conducting Proof-of-Concept studies in a quality and fast-paced manner.”
About AUR101-201 and the Phase II Study of AUR101 in Patients with Moderate to Severe Psoriasis
The purpose of the Phase II multi-center, blinded, placebo-controlled, three-arm study is to evaluate the clinical activity of AUR101 in patients with moderate to severe psoriasis. In two of the arms, AUR101 will be administered twice daily, at 400 mg PO BID and 600 mg PO BID, for 12 weeks. Patients in the third arm will receive matched blinded placebo in a double dummy fashion. The trial is listed at clinicaltrials.gov with identifier NCT04207801.
About Aurigene
Aurigene is a development stage biotech company engaged in discovery and clinical development of novel and best-in-class therapies to treat cancer and inflammatory diseases and a wholly owned subsidiary of Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY,NYSE: RDY). Aurigene is focused on precision- oncology, oral immune checkpoint inhibitors, and the Th-17 pathway. Aurigene currently has several programs from its pipeline in clinical development. Aurigene has also submitted an IND to DCGI, India for a Phase IIb/III trial of CA-170, a dual inhibitor of PD-L1 and VISTA, in non-squamous NSCLC. Additionally, Aurigene has multiple compounds at different stages of pre-clinical development. Aurigene has partnered with many large and mid-pharma companies in the United States and Europe and has 15 programs currently in clinical development. For more information, please visit Aurigene’s website at https://www.aurigene.com/.

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
CLIP
Signalling of multiple interleukin (IL)-17 family cytokines via IL-17 receptor A drives psoriasis-related inflammatory pathways
https://onlinelibrary.wiley.com/doi/10.1111/bjd.20090
M.A.X. Tollenaere,J. Hebsgaard,D.A. Ewald,P. Lovato,S. Garcet,X. Li,S.D. Pilger,M.L. Tiirikainen,M. Bertelsen,J.G. Krueger,H. Norsgaard,First published: 01 April 2021 https://doi.org/10.1111/bjd.20090Citations: 2Funding sources LEO Pharma A/S funded this study.Conflicts of interest M.A.X.T., J.H., D.A.E., P.L., S.D.P., M.L.T., M.B. and H.N. are employees of LEO Pharma. J.G.K. received grants paid to his institution from Novartis, Pfizer, Amgen, Lilly, Boehringer, Innovaderm, BMS, Janssen, AbbVie, Paraxel, LEO Pharma, Vitae, Akros, Regeneron, Allergan, Novan, Biogen MA, Sienna, UCB, Celgene, Botanix, Incyte, Avillion and Exicure; and personal fees from Novartis, Pfizer, Amgen, Lilly, Boehringer, Biogen Idec, AbbVie, LEO Pharma, Escalier, Valeant, Aurigene, Allergan, Asana, UCB, Sienna, Celgene, Nimbus, Menlo, Aristea, Sanofi, Sun Pharma, Almirall, Arena and BMS.Data Availability Statement The gene array dataset described in this publication has been deposited in NCBI’s Gene Expression Omnibus and is accessible through GEO Series accession number GSE158448 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE158448).
CLOP
https://www.drugdiscoverychemistry.com/Anti-Inflammatories/16
10:35 Small Molecule Inhibitors of RORgamma and IRAK4 for the Treatment of Autoimmune Disorders
 Susanta Samajdar, Ph.D., Director, Medicinal Chemistry, Aurigene Discovery Technologies Limited
Susanta Samajdar, Ph.D., Director, Medicinal Chemistry, Aurigene Discovery Technologies Limited
Although biologics such as anti-TNFα antibody are fairly successful in the treatment of autoimmune disorders, there is significant unmet need due to heterogeneity in diseases and lack of response to established therapies in some patients. While biologics typically target one cytokine signaling pathway, small molecule therapeutics directed towards intracellular target(s) can interfere in the signaling from multiple cytokines potentially leading to improved response. Development of small molecule oral inhibitors of IRAK4 and RORgamma to target TLR/IL-R and Th17 pathway respectively will be discussed.
PATENT
2448/CHE/2015 15.05.2015 IN
PATENT
PATENT
This application claims the benefit of Indian provisional application number 5641/CHE/2013 filed on 06th December 2013 which hereby incorporated by reference.
PATENT
- KOTRABASAIAH UJJINAMATADA, Ravi
- PANDIT, Chetan
2049005-13-0
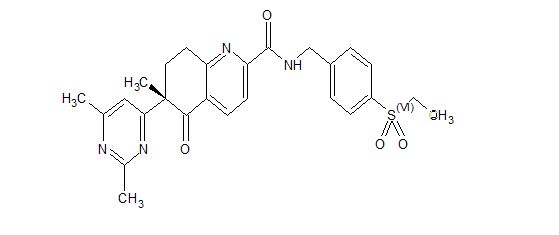
2-Quinolinecarboxamide, 6-(2,6-dimethyl-4-pyrimidinyl)-N-[[4-(ethylsulfonyl)phenyl]methyl]-5,6,7,8-tetrahydro-6-methyl-5-oxo-, (6S)-
Molecular Weight492.59, C26 H28 N4 O4 S
EXAMPLE
PATENT
CLIP
https://www.sciencedirect.com/science/article/abs/pii/S0223523419301011
2013239366 CA 170
///////////////////////AUR 101, AURIGENE, ROR, IL-17, PHASE 2, CDSCO, Ravi Ujjinamatada, KOTRABASAIAH UJJINAMATADA Ravi, PANDIT Chetan, AUR101-201, plaque-type psoriasis