
CEFADROXIL
- Molecular FormulaC16H17N3O5S
- Average mass363.388 Da
Cephos
- Molecular FormulaC16H19N3O6S
- Average mass381.404 Da
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Cefadroxil hemihydrate | J9CMF6461M | 119922-85-9 | AJAMDISMDZXITN-QXBGZBSVSA-N |
| Cefadroxil monohydrate | 280111G160 | 66592-87-8 | NBFNMSULHIODTC-CYJZLJNKSA-N |
| Cefadroxil sodium | SSZ6380I0I | 42284-83-3 | GQOVFIUWRATNJC-CYJZLJNKSA-M |
Cefadroxil is a cephalosporin antibiotic used in the treatment of various bacterial infections, such as urinary tract infections, skin and skin structure infections, and tonsillitis.
Cefadroxil (formerly trademarked as Duricef) is a broad-spectrum antibiotic of the cephalosporin type, effective in Gram-positive and Gram-negative bacterial infections. It is a bactericidal antibiotic.
It was patented in 1967 and approved for medical use in 1978.[1]
DURICEF (cefadroxil) is a semisynthetic cephalosporin antibiotic intended for oral administration. It is a white to yellowish-white crystalline powder. It is soluble in water and it is acid- stable. It is chemically designated as 5-Thia-l-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[amino(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-, monohydrate[6R- [6α,7β(R*)]]-. It has the formula C16H17N3O5S•H20 and the molecular weight of 381.40. It has the following structural formula:
 |
DURICEF (cefadroxil) film-coated tablets, 1 g, contain the following inactive ingredients: microcrystalline cellulose, hydroxypropyl methylcellulose, magnesium stearate, polyethylene glycol, polysorbate 80, simethicone emulsion, and titanium dioxide.
DURICEF (cefadroxil) for Oral Suspension contains the following inactive ingredients: FD&C Yellow No. 6, flavors (natural and artificial), polysorbate 80, sodium benzoate, sucrose, and xanthan gum.
DURICEF (cefadroxil) capsules contain the following inactive ingredients: D&C Red No. 28, FD&C Blue No. 1, FD&C Red No. 40, gelatin, magnesium stearate, and titanium dioxide.
SYN

IR spectrum of pure cefadroxil drug.
Synthesis Reference
Leonardo Marsili, “Substantially anhydrous crystalline cefadroxil and method for producing it.” U.S. Patent US5329001, issued April, 1978.
SYN
Antibiotics
R.S. Vardanyan, V.J. Hruby, in Synthesis of Essential Drugs, 2006
Cefadroxil
Cefadroxil, [6R-[6α,7β(R)]]-3-methyl-8-oxo-7-[[amino(4-hydroxyphenyl) acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.14), is an analog of cephalexin and differs only in the presence of a hydroxyl group in the fourth position of the phenyl ring of phenylglycine, and is synthesized by a scheme analogous to the scheme of cephradin synthesis [90–96].


Cefadroxil has a broad spectrum of antimicrobial action; it is active with respect to Gram-positive and Gram-negative microorganisms. Like all of the other drugs described above, it acts as a bactericide by disrupting the process of restoring the membranes of bacteria. Synonyms of this drug are bidocef, cefadril, duracef, ultracef, and others.
Cefadroxil
- ATC:J01DA09
- MW:363.39 g/mol
- CAS-RN:50370-12-2
- InChI Key:BOEGTKLJZSQCCD-UEKVPHQBSA-N
- InChI:InChI=1S/C16H17N3O5S/c1-7-6-25-15-11(14(22)19(15)12(7)16(23)24)18-13(21)10(17)8-2-4-9(20)5-3-8/h2-5,10-11,15,20H,6,17H2,1H3,(H,18,21)(H,23,24)/t10-,11-,15-/m1/s1
- EINECS:256-555-6
- LD50:>1.5 g/kg (M, i.v.); >10 g/kg (M, p.o.);
>1 g/kg (R, i.v.); >10 g/kg (R, p.o.);
>2 g/kg (dog, p.o.)
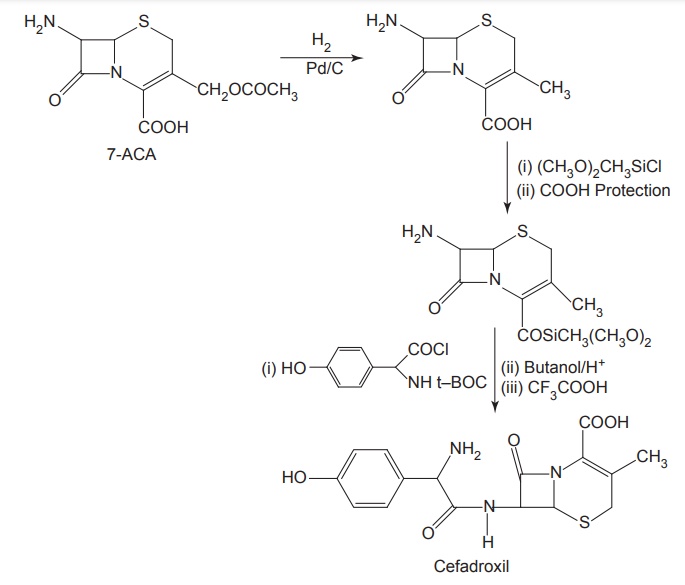
Synthesis
Substances
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 22252-43-3 | C8H10N2O3S | 7-amino-3-deacetoxycephalosporanic acid | 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-amino-3-methyl-8-oxo-, (6R-trans)- |
| 53487-89-1 | C13H15NO5 | d(–)-4-hydroxy-N-(2-methoxycarbonyl-1-methylethenyl)phenylglycine | Benzeneacetic acid, 4-hydroxy-α-[(3-methoxy-1-methyl-3-oxo-1-propenyl)amino]-, (R)- |
PATENT
https://patents.google.com/patent/WO2005042543A1/en
The compound of the above formula is cefprozil when A is -C=CH-CH3, cefatrizine when A is 1 H-1 ,2,3-triazole-4-yl-thiomethyl, and cefadroxii when A is -CH3. Conventionally, there have been known various processes for preparing oral cephalosporin antibiotics, such as cefprozil, cefatrizine, and cefadroxii, by reacting reactive derivatives of 4-hydroxyphenylglycine with 3-cephem compounds. For example, U.S. Patent No. 3,985,741 discloses a process for preparing a cefadroxii, which includes reacting 4-hydroxyphenylglycine and ethylchloroformate in



PAPER
Deshmukh, J. H.; Asian Journal of Chemistry 2010, V22(3), P1760-1768
Journal of the Chinese Chemical Society (Weinheim, Germany), 66(12), 1649-1657; 2019
Journal of the Indian Chemical Society, 93(6), 593-598; 2016
Biotechnology Letters, 34(9), 1719-1724; 2012
PATENT
WO 2011113486
By Gupta, Niranjan Lal et alFrom Indian, 184842, 30 Sep 2000
PAPER
European Journal of Organic Chemistry, (10), 1817-1820; 2001
PAPER
Organic Letters, 2(18), 2829-2831; 2000
The cephalosporin antibiotic Cefadroxil can be epimerized at the α-carbon of its amino acid side chain using pyridoxal as the mediator. By clathration with 2,7-dihydroxynaphthalene, the desired diastereomer can be selectively withdrawn from the equilibrating mixture of epimers. In this way, an asymmetric transformation of Cefadroxil can be accomplished. This opens the possibility of the production of Cefadroxil starting from racemic p-hydroxyphenylglycine, in contrast to the current industrial synthesis that employs the d-amino acid in enantiopure form.


AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Medical use
Cefadroxil is a first-generation cephalosporin antibacterial drug that is the para-hydroxy derivative of cephalexin, and is used similarly in the treatment of mild to moderate susceptible infections such as the bacterium Streptococcus pyogenes, causing the disease popularly called strep throat or streptococcal tonsillitis, urinary tract infection, reproductive tract infection, and skin infections.
Cefadroxil is used as an antibiotic prophylaxis before dental procedures, for patients allergic to penicillins.
Spectrum of bacterial resistance and susceptibility
Cefadroxil has a broad spectrum of activity and has been effective in treating bacteria responsible for causing tonsillitis, and infections of the skin and urinary tract. The following represents MIC susceptibility data for a few medically significant microorganisms.[2]
- Escherichia coli: 8 μg/ml
- Staphylococcus aureus: 1 – 2 μg/ml
- Streptococcus pneumoniae: ≤1 – >16 μg/ml
Side effects
The most common side effects of cefadroxil are diarrhea (which, less commonly, may be bloody), nausea, upset stomach, and vomiting. Other side effects include[3] rashes, hives, and itching.
Pharmacokinetics
Cefadroxil is almost completely absorbed from the gastrointestinal tract. After doses of 500 mg and 1 g by mouth, peak plasma concentrations of about 16 and 30 micrograms/ml, respectively, are obtained after 1.5 to 2.0 hours. Although peak concentrations are similar to those of cefalexin, plasma concentrations are more sustained. Dosage with food does not appear to affect the absorption of cefadroxil. About 20% of cefadroxil is reported to be bound to plasma proteins. Its plasma half-life is about 1.5 hours and is prolonged in patients with renal impairment.
Cefadroxil is widely distributed to body tissues and fluids. It crosses the placenta and appears in breast milk. More than 90% of a dose of cefadroxil may be excreted unchanged in the urine within 24 hours by glomerular filtration and tubular secretion; peak urinary concentrations of 1.8 mg/ml have been reported after a dose of 500 mg. Cefadroxil is removed by haemodialysis.
Dosage
Cefadroxil is given by mouth, and doses are expressed in terms of the anhydrous substance; 1.04 g of cefadroxil monohydrate is equivalent to about 1 g of anhydrous cefadroxil.
Veterinary use
It can be used for treating infected wounds on animals. Usually in powder form mixed with water, it has a color and smell similar to Tang. Given orally to animals, the amount is dependent on their weight and severity of infection.
References
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 493. ISBN 9783527607495.
- ^ “Cefadroxil, Free Acid Susceptibility and Minimum Inhibitory Concentration (MIC) Data” (PDF).
- ^ “Cefadroxil side effects”. Drugs.
 |
|
| Clinical data | |
|---|---|
| Trade names | Duricef |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682730 |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | plasma protein |
| Metabolism | unknown |
| Elimination half-life | 1.5 hours |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.397 |
| Chemical and physical data | |
| Formula | C16H17N3O5S |
| Molar mass | 363.39 g·mol−1 |
| 3D model (JSmol) | |
| |
|
////////////CEFADROXIL, цефадроксил , سيفادروكسيل , 头孢羟氨苄 , BL-S578; MJF-11567-3, BL S578, MJF 11567-3
[H][C@]12SCC(C)=C(N1C(=O)[C@H]2NC(=O)[C@H](N)C1=CC=C(O)C=C1)C(O)=O















