
Cenobamate
CAS: 913088-80-9
Chemical Formula: C10H10ClN5O2
Molecular Weight: 267.67
Related CAS #: 913088-80-9 913087-59-9
Synonym: YKP-3089; YKP3089; YKP3089; Cenobamate
IUPAC/Chemical Name: (R)-1-(2-chlorophenyl)-2-(2H-tetrazol-2-yl)ethyl carbamate
- 2H-Tetrazole-2-ethanol, α-(2-chlorophenyl)-, carbamate (ester), (αR)- (9CI)
- (1R)-1-(2-chlorophenyl)-2-(2H-tetrazol-2-yl)ethyl carbamate
- Carbamic acid (R)-(+)-1-(2-chlorophenyl)-2-(2H-tetrazol-2-yl)ethyl ester
- 2H-Tetrazole-2-ethanol, α-(2-chlorophenyl)-, 2-carbamate, (αR)-
Cenobamate, also known as YKP-3089, is a novel new antiepileptic drug candidate. Cenobamate showed broad-spectrum anticonvulsant activity. Cenobamate entered into clinical trials and was discontinued in 2015.
PATENT
WO 2006112685
SK HOLDINGS CO., LTD. [KR/KR]; 99 Seorin-dong Jongro-ku Seoul 110-110, KR
| CHOI, Yong-Moon; US |
| KIM, Choon-Gil; KR |
| KANG, Young-Sun; KR |
| YI, Han-Ju; KR |
| LEE, Hyun-Seok; KR |
| KU, Bon-Chul; KR |
| LEE, Eun-Ho; KR |
| IM, Dae-Joong; KR |
| SHIN, Yu-Jin; KR |
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2006112685
Patent
US 20100323410
PATENT
WO 2011046380
https://patentscope.wipo.int/search/en/detail.jsf%3Bjsessionid=9CF54FB903EC3DFB7B3237259E6419EB.wapp2?docId=WO2011046380&recNum=36&office=&queryString=&prevFilter=%26fq%3DOF%3AIL%26fq%3DICF_M%3A%22C07D%22&sortOption=Relevance&maxRec=1345
As disclosed in U. S. Patent Application Publication No. 2006/0258718 A1, carbamic acid (R)-1-aryl-2-tetrazolyl-ethyl esters (hereinafter referred to as “the carbamate compounds”) with anticonvulsant activity are useful in the treatment of disorders of the central nervous system, especially including anxiety, depression, convulsion, epilepsy, migraines, bipolar disorder, drug abuse, smoking, ADHD, obesity, sleep disorders, neuropathic pain, strokes, cognitive impairment, neurodegeneration, strokes and muscle spasms.
Depending on the position of N in the tetrazole moiety thereof, the carbamate compounds are divided into two positional isomers: tetrazole-1-yl (hereinafter referred to as “1N tetrazole”) and treatzole-2-yl (hereinafter referred to as “2N tetrazole”). The introduction of tetrazole for the preparation of the carbamate compounds results in a 1:1 mixture of the two positional isomers which are required to be individually isolated for pharmaceutical use.
Having chirality, the carbamate compounds must be in high optical purity as well as chemical purity as they are used as medications.
In this regard, U. S. Patent Application Publication No. 2006/0258718 A1 uses the pure enantiomer (R)-aryl-oxirane as a starting material which is converted into an alcohol intermediate through a ring-opening reaction by tetrazole in the presence of a suitable base in a solvent, followed by introducing a carbamoyl group into the alcohol intermediate. For isolation and purification of the 1N and 2N positional isomers thus produced, column chromatography is set after the formation of an alcohol intermediate or carbamate.
For use in the preparation, (R)-2-aryl-oxirane may be synthesized from an optically active material, such as substituted (R)-mandelic acid derivative, via various routes or obtained by asymmetric reduction-ring formation reaction of α-halo arylketone or by separation of racemic 2-aryl-oxirane mixture into its individual enantiomers. As such, (R)-2-aryl-oxirane is an expensive compound.
In addition, the ring-opening reaction of (R)-2-aryl-oxirane with tetrazole is performed at relatively high temperatures because of the low nucleophilicity of the tetrazole. However, the ring opening reaction includes highly likely risk of a runaway reaction because tetrazoles start to spontaneously degrade at 110 ~ 120℃.
In terms of a selection of reaction, as there are two reaction sites in each (R)-2-aryl-oxirane and tetrazole, the ring-opening reaction therebetween affords the substitution of 1N- or 2N-tetrazole at the benzyl or terminal position, resulting in a mixture of a total of 4 positional isomers. Therefore, individual positional isomers are low in production yield and difficult to isolate and purify.
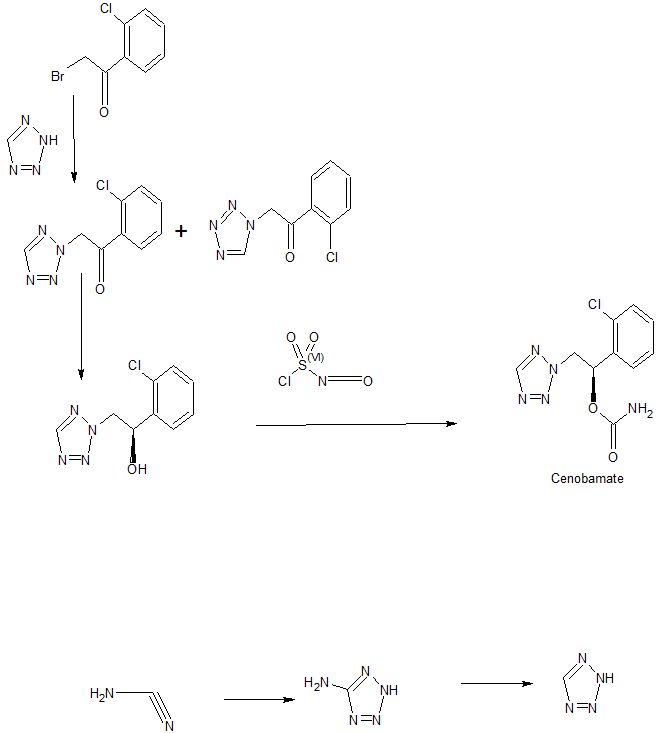
Preparation Example 1: Preparation of 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-1-yl)ethan-1-one
To a suspension of 2-bromo-2′-chloroacetophenone (228.3 g, 0.978 mol) and potassium carbonate (161.6 g, 1.170 mol) in acetonitrile (2000 mL) was added a 35 w/w% 1H-tetrazole dimethylformamide solution (215.1 g, 1.080 mol) at room temperature. These reactants were stirred for 2 h at 45℃ and distilled under reduced pressure to remove about 1500 mL of the solvent. The concentrate was diluted in ethyl acetate (2000 mL) and washed with 10% brine (3 x 2000 mL). The organic layer thus separated was distilled under reduced pressure to afford 216.4 g of an oily solid residue. To a solution of the solid residue in ethyl acetate (432 mL) was slowly added heptane (600 mL). The precipitate thus formed was filtered at room temperature and washed to yield 90.1 g (0.405 mol) of 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-1-yl)ethan-1-one (hereinafter referred to as 1N ketone ).
1H-NMR(CDCl 3) 8.87(s, 1H), d7.77(d, 1H), d7.39-7.62(m, 3H), d5.98(s, 2H)
Preparation Example 2: Preparation of 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-one
After the filtration of Preparation Example 1, the filtrate was concentrated and dissolved in isopropanol (100 mL), and to which heptane (400 mL) was then added to complete the crystallization. Filtering and washing at 5℃ afforded 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-one (hereinafter referred to as “2N ketone”) as a solid. 94.7 g (0.425 mol).
1H-NMR(CDCl 3) d8.62(s, 1H), d7.72(d, 1H), d7.35-7.55(m, 3H), d6.17(s, 2H)
PREPARATION EXAMPLE 3: Preparation of Alcohol Compound of (R)-Configuration by enantioselective enzymatic reduction via various oxidoreductases
The following four solutions were prepared as follows:
Competent Escherichia coli StarBL21(De3) cells (Invitrogen) were transformed with the expression constructs pET21-MIX coding for oxidoreductase SEQ ID NO 1. The Escherichia coli colonies transformed with the resulting expression constructs were then cultivated in 200 mL of LB medium (1% tryptone, 0.5 % yeast and 1% sodium chloride) with 50 micrograms/mL of ampicillin or 40 micrograms/mL of kanamycin, respectively, until an optical density of 0.5, measured at 550 nm, was achieved. The expression of the desired recombinant protein was induced by the addition of isopropylthiogalactoside (IPTG) to a concentration of 0.1 mM. After 16 hours of induction at 25 ℃ and 220 rpm, the cells were harvested and frozen at -20 ℃. In the preparation of the enzyme solutions, 30 g of cells were resuspended in 150 mL of triethanolamine buffer (TEA 100 nM, 2 mM MgCl2, 10% glycerol, pH 8) and homogenized in a high pressure homogenizer. The resultant enzyme solution was mixed with 150 mL glycerol and stored at -20℃.
RB791 cells ( E.coli genetic stock, Yale, USA) were transformed with the expression constructs pET21-MIX coding for oxidoreductase SEQ ID NO 2. The Escherichia coli colonies transformed with the resulting expression constructs were then cultivated in 200 mL of LB medium (1% tryptone, 0.5 % yeast and 1% sodium chloride) with 50 micrograms/mL of ampicillin or 40 micrograms/mL of kanamycin, respectively, until an optical density of 0.5, measured at 550 nm, was achieved. The expression of the desired recombinant protein was induced by the addition of isopropylthiogalactoside (IPTG) to a concentration of 0.1 mM. After 16 hours of induction at 25℃ and 220 rpm, the cells were harvested and frozen at -20℃. In the preparation of the enzyme solutions, 30 g of cells were resuspended in 150 mL of triethanolamine buffer (TEA 100 nM, 2 mM MgCl2, 10% glycerol, pH 8) and homogenized in a high pressure homogenizer. The resultant enzyme solution was mixed with 150 mL glycerol and stored at -20℃.
Enzyme solutions 3 was prepared in the same manner as described in Enzyme solution 1 except that expression constructs pET21-MIX coding for oxidoreductase SEQ ID NO 3 instead of expression constructs pET21-MIX coding for oxidoreductase SEQ ID NO 1 was used.
Enzyme solutions 4 was prepared in the same manner as described for enzyme solution 2 except that expression constructs pET21-MIX coding for oxidoreductase SEQ ID NO 4 instead of expression constructs pET21-MIX coding for oxidoreductase SEQ ID NO 2 was used.
Different oxidoreductases contained in each enzyme solutions 1 to 4 were examined as follows for the conversion of 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-1-yl)ethan-1-one (1N ketone) and 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-one (2N ketone) to the corresponding 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-1-yl)ethan-1-ol (hereinafter, referred to as 1N alcohol ) and 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-ol (hereinafter, referred to as “2N alcohol”), respectively.
160 ㎕ buffer (TEA 100 nM, 2 mM MgCl2, 10% glycerol, pH 8)
2 mg 1N ketone or 2N ketone
160 ㎕ buffer (TEA 100 nM, 2 mM MgCl2, 10% glycerol, pH 8)
2 mg 1N ketone or 2N ketone
350 ㎕ buffer (TEA 100 nM, 2 mM MgCl2, 10% glycerol, pH 8)
10 mg 1N ketone or 2N ketone
250 ㎕ 4-methyl-2-pentanol
50 ㎕ enzyme (oxidoreductase from Thermoanerobium brockii) solution for regeneration of cofactor
350 ㎕ buffer (TEA 100 nM, 2 mM MgCl2, 10% glycerol, pH 8
10 mg 1N ketone or 2N ketone
250 ㎕ 4-methyl-2-pentanol
50 ㎕ enzyme (oxidoreductase from Thermoanerobium brockii) solution for regeneration of cofactor
After 24h of incubating each reaction batch A, B, C and D, 1 mL of acetonitrile was added to each reaction batch which was centrifuged and transferred into a HPLC analysis vessel for enantiomeric excess and conversion. Conversion and ee-value of products are listed in Table 1 below calculated using the following equations:
Conversion Rate (%) = [(Area of Product)/(Area of Reactant + Area of Product)]x100
ee-value(%) = [(Area of R-Configuration – Area of S-Configuration)/(Area of R-Configuration + Area of S-Configuration)] x 100
Table 1 [Table 1]

PREPARATION EXAMPLE 4: Enzymatic reduction via oxidoreductase SEQ NO: 2
For the conversion of 1N/2N ketone to R-1N/R-2N alcohol, 30㎕ of the enzyme solution 2 containing the oxidoreductase SEQ NO: 2 were added to a mixture of 300㎕ of a buffer (100 mM TEA, pH 8, 1mM MgCl2, 10% glycerol), 100mg of a mixture of 1N ketone and 2N ketone (1N:2N=14%:86%), 0.04mg NADP and 300㎕ 2-butanol. The reaction mixture was incubated at room temperature under constant thorough mixing. After 48 hours, more than 98% of the ketones were reduced to an alcohol mixture of the following composition(R-2N alcohol 80%; S-2N alcohol 0%; R-1N alcohol 20%, S-1N alcohol 0%; 1N ketone 0%; 2N ketone 0%).
After general work up and recrystallization with ethyl acetate/hexane, optically pure alcohols were obtained as below:
(R)-1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-1-yl)ethan-1-ol (1N alcohol
1H-NMR(CDCl 3) d8.74(s, 1H), d7.21-7.63(m, 4H), d5.57(m, 1H), d4.90(d, 1H), d4.50(d, 1H), d3.18(d, 1H);
(R)-1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-ol (2N alcohol)
1H-NMR(CDCl 3) d8.55(s, 1H), d7.28-7.66(m, 4H), d5.73(d, 1H), d4.98(d, 1H), d4.83(d, 1H), d3.38(br, 1H).
Preparation Example 5: Preparation of Carbamic Acid (R)-1-(2-Chlorophenyl)-2-(tetrazol-2-yl)ethyl ester
50ml of the enzyme solution 2 containing the oxidoreductase SEQ NO: 2 were added to a mixture of 250ml of a buffer (100 mM TEA, pH 8, 1mM MgCl2, 10% glycerol), 50g (225mmol) of 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-one(2N ketone), 4mg NAD, 300 ml of 2-propanol and 150mL of butyl acetate. The reaction mixture was stirred at room temperature. After 48 hours more than 98% of 2N ketone was reduced to corresponding (R)-1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-ol (R-2N alcohol) with >99%ee values. To this resulting mixture, 500mL of ethyl acetate was added. After being separated, the organic layer thus formed was washed with 10% brine (3 x 500mL). The organic layer thus formed was dried over magnesium sulfate and filtered and the filtrate was distilled under reduced pressure to give 50.4g (224 mmol) of 1-(2-chlorophenyl)-2-(1,2,3,4-tetrazol-2-yl)ethan-1-ol (R-2N alcohol, optical purity 99.9%) as an oily residue. To this resulting crude product, 450mL of tetrahydrofuran was added. After cooling to -15℃, 38g (267mmol) of chlorosulfonyl isocyanate was slowly added and stirred at -10℃ for 2 h. The slow addition of water induced termination of the reaction. The resulting solution was concentrated under reduced pressure until about 300 mL of the solvent was removed. The concentrate was diluted with 600mL of ethyl acetate and washed with 10% brine (3 x 500 mL). The organic layer was concentrated under reduced pressure and the concentrate was dissolved in isopropanol (90 mL) to which heptane (180 mL) was slowly added, leading to the completion of crystallization. The precipitate thus obtained was filtered and washed to afford 51.8 g (194 mmol) of carbamic acid (R)-1-(2-chlorophenyl)-2-(tetrazol-2-yl)ethyl ester (optical purity 99.9%).
1H-NMR(Acetone-d 6) d8.74(s, 1H), d7.38-7.54(m, 4H), d6.59(m, 1H), d6.16(Br, 2H), d4.90(d, 1H), d5.09(m, 2H)
As described hitherto, carbamate compounds with high optical and chemical purity can be produced with an economical benefit in accordance with the present invention.
Although the preferred embodiments of the present invention have been disclosed for illustrative purposes, those skilled in the art will appreciate that various modifications, additions and substitutions are possible, without departing from the scope and spirit of the invention as disclosed in the accompanying claims.
SEQ ID NO 1: Oryctolagus cuniculus from rabbit DSMZ 22167
SEQ ID NO 2: Candida magnoliae DSMZ 22052 protein sequence carbonyl reductase
SEQ ID NO 3: Candida vaccinii CBS7318 protein sequence carbonyl reductase
SEQ ID NO 4: Candida magnoliae CBS6396 protein sequence carbonyl reductase
SEQ ID NO 5: Oryctolagus cuniculus from rabbit DSMZ 22167
SEQ ID NO 6: Candida magnoliae DSMZ 22052 nucleic acid sequence carbonyl reductase
SEQ ID NO 7: Candida vaccinii CBS7318 nucleic acid sequence carbonyl reductase
SEQ ID NO 8: Candida magnoliae CBS6396 nucleic acid sequence carbonyl reductase
Clip
https://www.linkedin.com/company/sk-life-science-inc./
https://www.sklifescienceinc.com/
SK life science announces FDA acceptance of NDA submission for cenobamate, an investigational antiepileptic drug PDUFA date set for November 21, 2019 Fair Lawn, New Jersey, February 4, 2019 – SK Life Science, Inc., a subsidiary of SK Biopharmaceuticals Co., Ltd., an innovative biopharmaceutical company focused on developing and bringing to market treatments for central nervous system (CNS) disorders, announced today that the U.S. Food and Drug Administration (FDA) has accepted the filing of its New Drug Application (NDA) for cenobamate. Cenobamate, an investigational antiepileptic drug for the potential treatment of partial-onset seizures in adult patients, is the first molecule discovered and developed from inception through to the submission of an NDA without partnering or out-licensing from a Korean pharmaceutical company.
SK life science plans to commercialize cenobamate independently. The NDA submission is based on data from pivotal trials that evaluated the efficacy and safety of cenobamate. Results from the clinical trial program, which enrolled more than 1,900 patients, have been presented at medical conferences including the American Academy of Neurology (AAN) and the American Epilepsy Society (AES) Annual Meetings. “The FDA’s acceptance of our NDA filing is a critical step toward our goal of introducing a new treatment option for people with uncontrolled epilepsy,” said Marc Kamin, M.D., chief medical officer at SK life science. “We look forward to working with the FDA during their review of our data on cenobamate.” Despite the availability and introduction of many new AEDs, overall treatment outcomes for people with epilepsy have not improved in 20 years
1 and the CDC states that nearly 60 percent of people with epilepsy are still experiencing seizures, showcasing a great unmet need for patients and their families. 2 Additionally, while some patients may experience a reduction in seizure frequency with current treatments, they continue to live with seizures.
2 The impact of continued seizures can be debilitating and life-altering and the complications of epilepsy can include depression and anxiety, cognitive impairment and SUDEP (sudden unexpected death in epilepsy).
3 About Epilepsy Epilepsy is a common neurological disorder characterized by seizures.
4 There are approximately 3.4 million people in the U.S. living with epilepsy, and approximately 65 million worldwide.
5 The majority of people with epilepsy (60%) have partial-onset seizures, which are located in just one part of the brain.
6 People with epilepsy are also at risk for accidents and other health complications including falling, drowning, car accidents, depression and anxiety and SUDEP. 3
About Cenobamate Cenobamate (YKP3089) was discovered by SK Biopharmaceuticaals and SK life science and is being investigated for the potential treatment of partial-onset seizures in adult patients. Cenobamate’s mechanism of action is not fully understood, but it is believed to work through two separate mechanisms: enhancing inhibitory currents through positive modulation of GABA-A receptors and decreasing excitatory currents by inhibiting the persistent sodium current. Global trials for adults with partial-onset seizures are ongoing to evaluate cenobamate safety.
Additional clinical trials are investigating cenobamate safety and efficacy in other seizure types. The U.S. Food and Drug Administration (FDA) accepted the filing of the New Drug Application for cenobamate for the potential treatment of partial-onset seizures in adults in February 2019. Cenobamate is not approved by the FDA or any other regulatory authorities. Safety and efficacy have not been established. About SK life science SK Life Science, Inc., a subsidiary of SK Biopharmaceuticals, Co., Ltd., is focused on developing and commercializing treatments for disorders of the central nervous system (CNS).
Both are a part of the global conglomerate SK Group, the second largest company in Korea. SK life science is located in Fair Lawn, New Jersey. We have a pipeline of eight compounds in development for the treatment of CNS disorders including epilepsy, sleep disorder and attention deficit hyperactivity disorder, among others. The first product the company is planning to commercialize independently is cenobamate (YKP3089), an investigational compound for the potential treatment of partial-onset seizures in adult patients, currently in a Phase 3 global clinical trial.
For more information, visit SK life science’s website at www.SKLifeScienceInc.com.
For more information, visit SK Biopharmaceuticals’ website at www.skbp.com/eng. —-
1. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. https://www.ncbi.nlm.nih.gov/pubmed/29279892 Published online December 26, 2017.
2. Center for Disease Control and Prevention. Active Epilepsy and Seizure Control in Adults — United States, 2013 and 2015. https://www.cdc.gov/mmwr/volumes/67/wr/mm6715a1.htm?s_cid=mm6715a1 Accessed December 27, 2018.
3. Epilepsy Foundation. Staying Safe. https://www.epilepsy.com/learn/seizure-first-aid-and-safety/staying-safe Accessed November 20, 2018.
4. Epilepsy Foundation. What Is Epilepsy? https://www.epilepsy.com/learn/about-epilepsy-basics/what-epilepsy Accessed November 20, 2018.
5. Epilepsy Foundation. Facts about Seizures and Epilepsy. https://www.epilepsy.com/learn/about-epilepsybasics/facts-about-seizures-and-epilepsy Accessed November 20, 2018.
6. National Institute of Neurological Disorders and Stroke. The Epilepsies and Seizures: Hope through Research. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Epilepsies-andSeizures-Hope-Through#3109_9 Accessed November 20, 2018.
REFERENCES
1: Mula M. Emerging drugs for focal epilepsy. Expert Opin Emerg Drugs. 2013
Mar;18(1):87-95. doi: 10.1517/14728214.2013.750294. Epub 2012 Nov 26. Review.
PubMed PMID: 23176519.
2: Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress
report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT
IX). Epilepsy Res. 2009 Jan;83(1):1-43. doi: 10.1016/j.eplepsyres.2008.09.005.
Epub 2008 Nov 12. PubMed PMID: 19008076.
/////////////YKP-3089, YKP3089, YKP3089, Cenobamate
NC(O[C@H](C1=CC=CC=C1Cl)CN2N=CN=N2)=O

















