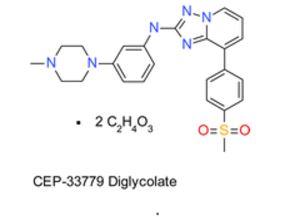
CEP-33779, CEP33779
CAS 1257704-57-6
Chemical Formula: C24H26N6O2S
Molecular Weight: 462.57
Elemental Analysis: C, 62.32; H, 5.67; N, 18.17; O, 6.92; S, 6.93
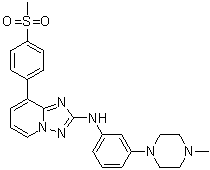
N-(3-(4-methylpiperazin-1-yl)phenyl)-8-(4-(methylsulfonyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-amine
PRECLINICAL Treatment of Rheumatoid Arthritis, Agents for Colorectal Cancer Therapy Systemic Lupus Erythematosus,
Jak2 Inhibitors
| Matthew A. Curry, Bruce D. Dorsey, Benjamin J. Dugan, Diane E. Gingrich, Eugen F. Mesaros, Karen L. Milkiewicz, | |
| Applicant | Cephalon, Inc. |
Worldwide Discovery Research, Cephalon, Inc., 145 Brandywine Parkway, West Chester, Pennsylvania 19380, United States



Benjamin J. Dugan received a B.S. degree in Chemistry from the University of Delaware in 1993 under the tutelage of the late Dr. Cynthia McClure. He began his career at FMC Corporation in the agricultural products division. In 2006, he moved to Cephalon, Inc., acquired by Teva Pharmaceutical Industries Ltd. in 2011, and engaged in oncology research focused on small molecule, ATP competitive, kinase inhibitors culminating with the discovery of CEP-33779. He is currently a Research Scientist focused on the development of novel, bioactive small molecules for treatment of central nervous system disorders.
Members of the Cephalon research team that discovered CEP-5214 and CEP-7055 include (from left) Hudkins, Thelma S. Angeles, Bruce A. Ruggeri, and Diane E. Gingrich. CEPHALON PHOTO


Lupus (systemic lupus erythematosus, SLE) is a chronic autoimmune disease characterized by the presence of activated T and B cells, autoantibodies and chronic inflammation that attacks various parts of the body including the joints, skin, kidneys, CNS, cardiac tissue and blood vessels. In severe cases, antibodies are deposited in the cells (glomeruli) of the kidneys, leading to inflammation and possibly kidney failure, a condition known as lupus nephritis.
Although the cause of lupus remains unknown, manifestations of the disease have been linked to genetic polymorphisms, environmental toxins and pathogens (Morel;
Fairhurst, Wandstrat et al. 2006). In addition, gender, hormonal influences and cytokine dysregulation have been tightly linked to the development of lupus (Aringer and Smolen 2004; Smith-Bouvier, Divekar et al. 2008). Lupus affects nine times as many women as men. It may occur at any age, but appears most often in people between the ages of 10 and 50 years. African Americans and Asians are affected more often than people from other races.
There is no cure for lupus. Current treatments for lupus are aimed at controlling symptoms and are limited to toxic and immunosuppressive agents with severe side-effects such as high dose glucocorticoids and/or hydroxchloroquine. Severe disease (e.g., patients that have signs of renal involvement) require more aggressive drugs including
mycophenolate mofetil (MMF), azathioprine (AZA) and/or cyclophosphamide (CTX) (Bertsias and Boumpas 2008). CTX, AZA and MMF are very toxic and
immunosuppressive, and only 50% of treated patients enter complete remission, with relapse rates up to 30% over a 2-year period.
Memory B cells, and more important, long-lived plasma cells (LL-PCs) which differentiate from memory B cells, are key cell types involved in lupus (Neubert, Meister et al. 2008; Sanz and Lee 2010). Long-lived plasma cells synthesize and secrete large quantities of high-affinity isotype switched antibodies (Meister, Schubert et al. 2007;
Muller, Dieker et al. 2008). Circulating antinuclear antibodies (ANAs) increase the chances of antibody depositing onto self tissues, forming immune-complexes and eventually leading to tissue destruction, epitope spreading and involvement of other organ systems. LL-PCs are commonly found to be chemo- and radio-resistant, over expressing various heat shock proteins and drug pumps (Obeng, Carlson et al. 2006; Neubert, Meister et al. 2008). In addition, LL-PCs primarily reside in the bone marrow where they are protected from current lupus therapies such as cyclophosphamide and glucocorticoids.
A need exists for new treatments for lupus, including lupus nephritis. A need particularly exists for lupus treatments that can target and reduce LL-PCs.
CEP-33779 is a highly selective, orally active, small-molecule inhibitor of JAK2. CEP-33779 induced regression of established colorectal tumors, reduced angiogenesis, and reduced proliferation of tumor cells. Tumor regression correlated with inhibition of STAT3 and NF-κB (RelA/p65) activation in a CEP-33779 dose-dependent manner. The ability of CEP-33779 to suppress growth of colorectal tumors by inhibiting the IL-6/JAK2/STAT3 signaling suggests a potential therapeutic utility of JAK2 inhibitors in multiple tumors types, particularly those with a strong inflammatory component.
{[8-(4-Methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-[3-(4-methyl-piperazin-1-yl)-phenyl]-amine} (1)
PATENT
WO 2010141796
https://www.google.com/patents/WO2010141796A3?cl=en
Example 35 [8-(4-Methanesulfonyl-phenyl)-[ 1 ,2,4]triazolo[ 1 ,5-a]pyridin-2-yl]-[3-(4-methyl-piperazin-
1 -yl)-phenyl]-amine
35 a) l-(3-Bromo-phenyl)-4-methyl-piperazine was prepared from l-(3-bromo-phenyl)- piperazine (1.33 g, 5.52 mmol) in a manner analogous to Step 32a. The reaction product was isolated as a pale yellow oil (1.4 g, 100%). 1H NMR (400 MHz, CDCl3, δ, ppm): 7.10 (dd, J=8.2, 8.2 Hz, IH), 7.04 (dd, J=2.1, 2.1 Hz, IH), 6.95 (ddd, J=I. S, 1.7, 0.7 Hz, IH), 6.83 (ddd, J=8.3, 2.4, 0.6 Hz, IH), 3.23-3.18 (m, 4H), 2.58-2.54 (m, 4H), 2.35 (s, 3H). MS = 255, 257 (MH)+. 35b) [8-(4-Methanesulfonyl-phenyl)-[ 1 ,2,4]triazolo[ 1 ,5-a]pyridin-2-yl]-[3-(4-methyl- piperazin-l-yl)-phenyl]-amine was prepared from 8-(4-methanesulfonyl-phenyl)- [l,2,4]triazolo[l,5-a]pyridin-2-ylamine (75.0 mg, 0.260 mmol) and l-(3-bromo-phenyl)-4- methyl-piperazine (80.0 mg, 0.314 mmol) with 2,2′-bis-dicyclohexylphosphanyl-biphenyl (30.0 mg, 0.0549 mmol) as the ligand in a manner analogous to Step 2d and was isolated as a yellow solid (0.072 g, 60%).
MP = 232-234 0C.
1H NMR (400 MHz, CDCl3, δ, ppm): 8.49 (d, J=I 2 Hz, IH), 8.25 (d, J=I .5 Hz, 2H), 8.08 (d, J=I .9 Hz, 2H), 7.65 (d, J=I .1 Hz, IH), 7.38 (s, IH), 7.27-7.20 (m, IH), 7.04-6.95 (m, 2H), 6.84 (s, IH), 6.60 (d, J=8.0 Hz, IH), 3.30-3.25 (m, 4H), 3.10 (s, 3H), 2.63-2.58 (m, 4H), 2.38 (s, 3H).
MS = 463 (MH)+.
PATENT
WO 2012078504
PATENT
WO 2012078574
https://google.com/patents/WO2012078574A2?cl=da
COMPOUND A is a JAK2 inhibitor with the chemical name [8-(4-methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-[3-(4-methyl-piperazin-1-yl)-phenyl]-amine. COMPOUND A has the following structure:

COMPOUND A
COMPOUND A was prepared in a manner analogous to the five-step method described below (see Example 35 of International Application No. PCT/US10/37363):
Step 1 : To a solution of 1-(3-bromo-phenyl)-piperazine (about 1 g) and acetic acid (about 0.4 mL) in methanol (about 25 mL) is added 37% formaldehyde in water/methanol (about 56.7:37:6.3, water:formaldehyde:methanol; about 5 mL). The mixture is stirred at room temperature for about 18 hours. The suspension is cooled to about 5°C in an ice/water bath and sodium cyanoborohydride (about 5 g) is added in small portions. The mixture is stirred and warmed to room temperature for about 18 hours. The mixture is slowly poured into saturated aqueous ammonium chloride (about 200 mL) and stirred for about 1 hour. The mixture is extracted with dichloromethane (3 x about 75 mL). The combined organic layers are dried over magnesium sulfate, filtered and evaporated. The material is placed under high vacuum for about 18 hours to yield 1-(3-bromo-phenyl)-4-methyl-piperazine as a pale yellow oil (about 1 g). 1H NMR (400 MHz, CDCl3, δ, ppm): 7.10 (dd, J=8.2, 8.2 Hz, 1H), 7.04 (dd, J=2.1, 2.1 Hz, 1H), 6.95 (ddd, J=7.8, 1.7, 0.7 Hz, 1H), 6.83 (ddd, J=8.3, 2.4, 0.6 Hz, 1H), 3.23-3.18 (m, 4H), 2.58-2.54 (m, 4H), 2.35 (s, 3H). MS = 255, 257 (MH)+.
Step 2: To a solution of 3-bromo-pyridin-2-ylamine (about 10 g) in 1,4-dioxane (about 100 mL) is added dropwise ethoxycarbonyl isothiocyanate (about 7 mL). The mixture is stirred under an atmosphere of nitrogen for about 18 hours. The volatiles are evaporated to yield a waxy solid. The recovered material is triturated with hexane (about 250 mL). N-(3-bromo-2-pyridinyl)-N’-carboethoxy-thiourea is isolated and used without further purification. 1H NMR (400 MHz, (D3C)2SO, δ, ppm): 11.46 (s, 1H), 11.43 (s, 1H), 8.49 (dd, J=4.6, 1.5 Hz, 1H), 8.18 (dd, J=8.0, 1.5 Hz, 1H), 7.33 (dd, J=8.0, 4.7 Hz, 1H), 4.23 (q, J=7.1 Hz, 2H), 1.27 (t, J=7.2 Hz, 3H). MS = 215 (MH)+.
Step 3: To a stirred suspension of hydroxylamine hydrochloride (about 17 g) and Ν,Ν-diisopropylethylamine (about 26 mL) in a mixture of methanol (about 70 mL) and
ethanol (about 70 mL) is added N-(3-bromo-2-pyridinyl)-N’-carboethoxy-thiourea. The mixture is stirred for about 2 hours at room temperature then heated to about 60°C for about 18 hours. The suspension is cooled to room temperature, filtered and rinsed with methanol, water then methanol. 8-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine is isolated as an off-white solid (about 8 g). 1H NMR (400 MHz, (D3C)2SO, δ, ppm): 8.58 (d, J=6.4 Hz, 1H), 7.73 (d, J=7.6 Hz, 1H), 6.80 (t, J=7.0 Hz, 1H), 6.25 (s, 2H). MS = 213, 215 (MH)+.
Step 4: An oven dried tube is charged with palladium acetate (about 0.2 g) and triphenylphosphine (about 0.6 g). The tube is evacuated under high vacuum and backflushed under a stream of nitrogen for about 5 minutes. A suitable solvent such as
1,4-dioxane (about 10 mL) is added and the mixture is stirred under nitrogen for a suitable time (e.g., for about 10 minutes). 8-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine (about 0.75 g), (4-methylsulfonylphenyl)boronic acid (about 1 g), a suitable solvent, such as N,N-dimethylformamide (about 10 mL) and a suitable base, such as about 1.5 M of sodium carbonate in water (about 10 mL) are added. The mixture is stirred for about 2 minutes at room temperature under nitrogen then the tube is sealed and heated at about 80°C for about 18 hours. The mixture is transferred to a round bottom flask and the volatiles are evaporated under reduced pressure. The product is isolated in a suitable manner. For example, water (about 100 mL) may be added and the mixture stirred. The solid may then be collected by filtration, and optionally rinsed with water, air dried, triturated with ether/dichloromethane (about 4: 1; about 10 mL), filtered and rinsed with ether. 8-(4-methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine is isolated as a tan solid (about 0.6 g). MP = 236-239 °C. 1H NMR (400 MHz, (D3C)2SO, δ, ppm): 8.63 (d, J=6.3 Hz, 1H), 8.38 (d, J=7.9 Hz, 2H), 8.03 (d, J=7.9 Hz, 2H), 7.84 (d, J= 7.3 Hz, 1H), 7.03 (t, J=7.0 Hz, 1H), 6.21 (br s, 2H), 3.28 (s, 3H). MS = 289 (MH)+.
Step 5: To an oven dried tube is added palladium acetate (about 10 mg) and 2,2′-bis-dicyclohexylphosphanyl-biphenyl (about 30 mg), 8-(4-methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine (about 75 mg), 1-(3-bromo-phenyl)-4-methyl-piperazine (about 80 mg), a suitable base, such as cesium carbonate (about 270 mg) and a suitable solvent, such as 1,4-dioxane (about 5 mL). The tube is evacuated and backflushed with nitrogen three times. The tube is sealed and heated at about 80°C for about 72 hours. The mixture is cooled to room temperature and the product isolated in a suitable manner.
For example, the cooled mixture may be diluted with dichloromethane (about 10 mL), filtered through a plug of diatomaceous earth, rinsed with dichloromethane and evaporated. The material may then be purified, e.g., via chromatography, e.g., utilizing an ISCO automated purification apparatus (e.g., amine modified silica gel column 5%→100% ethyl acetate in hexanes). [8-(4-Methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-[3-(4-methyl-piperazin-1-yl)-phenyl]-amine (i.e., COMPOUND A) is isolated as a yellow solid (about 0.07 g). MP = 232-234 °C. 1H NMR (400 MHz, CDCl3, δ, ppm): 8.49 (d, J=7.2 Hz, 1H), 8.25 (d, J=7.5 Hz, 2H), 8.08 (d, J=7.9 Hz, 2H), 7.65 (d, J=7.7 Hz, 1H), 7.38 (s, 1H), 7.27-7.20 (m, 1H), 7.04-6.95 (m, 2H), 6.84 (s, 1H), 6.60 (d, J=8.0 Hz, 1H), 3.30-3.25 (m, 4H), 3.10 (s, 3H), 2.63-2.58 (m, 4H), 2.38 (s, 3H). MS = 463 (MH)+.
PATENT
WO 2015089153
https://www.google.com/patents/WO2015089153A1?cl=un
This disclosure relates to a l,2,4 riazolo[l,5a]pyridine derivative, [8-(4 methanesulfonyl-phenyl)-[ 1 ,2,4]triazoio[1 ,5-a]pyridin-2-yl]-[3-(4-methyl-piperazin- 1 -yl phenyl] -amine, re g structure:

or a pharmaceutical salt thereof, and its use in the treatment of multiple sclerosis.
Compound A is a potent, orally active, small molecule inhibitor of JA 2. See, e.g..International Application No. PCT/USlO/37363, U.S. Patent Nos. 8,501,936 and ,633,173, and U.S. Published Patent Application Nos. 2013/0267535 and 2014/0024655, each of which is incorporated by reference herein. Compound A can be prepared, for example, using methods analogous to Example 35 of International Application No.PCT/US 10/37363.
PAPER
A Selective, Orally Bioavailable 1,2,4-Triazolo[1,5-a]pyridine-Based Inhibitor of Janus Kinase 2 for Use in Anticancer Therapy: Discovery of CEP-33779
Abstract

Members of the JAK family of nonreceptor tyrosine kinases play a critical role in the growth and progression of many cancers and in inflammatory diseases. JAK2 has emerged as a leading therapeutic target for oncology, providing a rationale for the development of a selective JAK2 inhibitor. A program to optimize selective JAK2 inhibitors to combat cancer while reducing the risk of immune suppression associated with JAK3 inhibition was undertaken. The structure–activity relationships and biological evaluation of a novel series of compounds based on a 1,2,4-triazolo[1,5-a]pyridine scaffold are reported. Para substitution on the aryl at the C8 position of the core was optimum for JAK2 potency (17). Substitution at the C2 nitrogen position was required for cell potency (21). Interestingly, meta substitution of C2-NH-aryl moiety provided exceptional selectivity for JAK2 over JAK3 (23). These efforts led to the discovery of CEP-33779 (29), a novel, selective, and orally bioavailable inhibitor of JAK2.
[8-(4-Methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-[3-(4-methyl-piperazin-1-yl)-phenyl]-amine (29)
PAPER
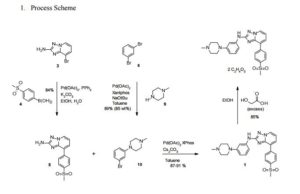
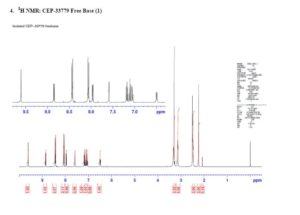
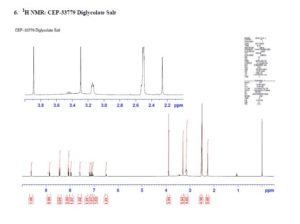
An Improved Synthesis of the Free Base and Diglycolate Salt of CEP-33779; A Janus Kinase 2 Inhibitor
Abstract

CEP-33779 is a triazole that has been reported to show highly selective inhibition of Janus kinase 2 (JAK2). An efficient process to form CEP-33779 will be presented that uses multiple palladium couplings to provide the drug substance in a convergent manner. The existing medicinal chemistry route was modified to avoid chromatographic purification, improve safety, and utilize palladium ligands which are available in quantities amenable to scale-up. Challenges faced during the development of the new process included optimization of conditions for Buchwald–Hartwig and Suzuki couplings, control of homocoupled impurities and removal of residual palladium. In addition, a screen of conditions to form a diglycolate salt of the parent compound are also presented.
REFERENCES
1: Dugan BJ, Gingrich DE, Mesaros EF, Milkiewicz KL, Curry MA, Zulli AL, Dobrzanski P, Serdikoff C, Jan M, Angeles TS, Albom MS, Mason JL, Aimone LD, Meyer SL, Huang Z, Wells-Knecht KJ, Ator MA, Ruggeri BA, Dorsey BD. A selective, orally bioavailable 1,2,4-triazolo[1,5-a]pyridine-based inhibitor of Janus kinase 2 for use in anticancer therapy: discovery of CEP-33779. J Med Chem. 2012 Jun 14;55(11):5243-54. doi: 10.1021/jm300248q. Epub 2012 May 18. PubMed PMID: 22594690.
2: Tagoe C, Putterman C. JAK2 inhibition in murine systemic lupus erythematosus. Immunotherapy. 2012 Apr;4(4):369-72. doi: 10.2217/imt.12.20. PubMed PMID: 22512630.
3: Seavey MM, Lu LD, Stump KL, Wallace NH, Hockeimer W, O’Kane TM, Ruggeri BA, Dobrzanski P. Therapeutic efficacy of CEP-33779, a novel selective JAK2 inhibitor, in a mouse model of colitis-induced colorectal cancer. Mol Cancer Ther. 2012 Apr;11(4):984-93. doi: 10.1158/1535-7163.MCT-11-0951. Epub 2012 Feb 14. PubMed PMID: 22334590.
4: Lu LD, Stump KL, Wallace NH, Dobrzanski P, Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Mason JL, Ator MA, Dorsey BD, Ruggeri BA, Seavey MM. Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. J Immunol. 2011 Oct 1;187(7):3840-53. doi: 10.4049/jimmunol.1101228. Epub 2011 Aug 31. PubMed PMID: 21880982.
5: Stump KL, Lu LD, Dobrzanski P, Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Ator MA, Dorsey BD, Ruggeri BA, Seavey MM. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis Res Ther. 2011 Apr 21;13(2):R68. doi: 10.1186/ar3329. PubMed PMID: 21510883; PubMed Central PMCID: PMC3132063.
/////////////CEP-33779, CEP33779, CEP 33779, 1257704-57-6, PRECLINICAL, TEVA, Rheumatoid Arthritis, Colorectal Cancer Therapy, Systemic Lupus Erythematosus,
Jak2 Inhibitors
O=S(C1=CC=C(C2=CC=CN3C2=NC(NC4=CC=CC(N5CCN(C)CC5)=C4)=N3)C=C1)(C)=O