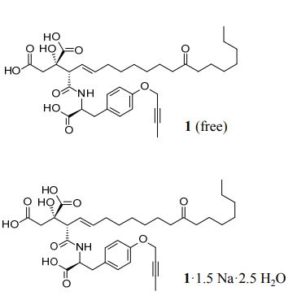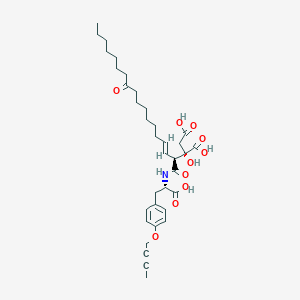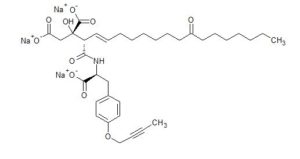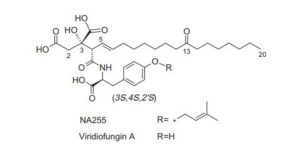
CH4630808, CH-4630808, NA-808
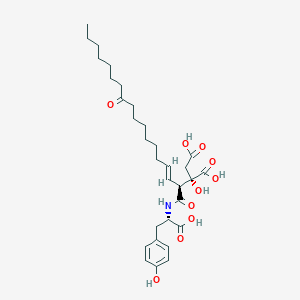
(2S)-2-[(E,2S)-1-[[(1S)-2-(4-but-2-ynoxyphenyl)-1-carboxyethyl]amino]-1,11-dioxooctadec-3-en-2-yl]-2-hydroxybutanedioic acid
| Molecular Formula: | C35H49NO10 |
|---|---|
| Molecular Weight: | 643.774 g/mol |
Cas 827034-92-4 DOUBLE BOND E, SP ROT (-)
CAS 744208-75-1 E Z NOT DEFINED
- D-erythro-Pentonic acid, 5-[[(1S)-2-[4-(2-butynyloxy)phenyl]-1-carboxyethyl]amino]-3-C-carboxy-2,4,5-trideoxy-5-C-oxo-4-[(1E)-9-oxo-1-hexadecenyl]- (9CI)
- 5-[[(1S)-2-[4-(2-Butyn-1-yloxy)phenyl]-1-carboxyethyl]amino]-3-C-carboxy-2,4,5-trideoxy-5-C-oxo-4-[(1E)-9-oxo-1-hexadecen-1-yl]-D-erythro-pentonic acid
- D-erythro-Pentonic acid, 5-[[(1S)-2-[4-(2-butyn-1-yloxy)phenyl]-1-carboxyethyl]amino]-3-C-carboxy-2,4,5-trideoxy-5-C-oxo-4-[(1E)-9-oxo-1-hexadecen-1-yl]-
Chugai Pharmaceutical (Originator)
Trisodium Der ,CAS 1799542-36-1, SP ROT (-), MW 709.7097, MF C35 H46 N O10 . 3 Na, Trisodium (2S)-2-[(2S,3E)-1-([(1S)-2-[4-(but-2-yn-1-yloxy)phenyl]-1-carboxylatoethyl]amino)-1,11-dioxooctadec-3-en-2-yl]-2-hydroxybutanedioate
SIMILAR
PAPER
https://www.sciencedirect.com/science/article/pii/S0960894X12013741
Bioorganic & Medicinal Chemistry Letters


Scheme 3. Reagents and conditions: (a) TBDPSCl, imidazole, DMF, rt; (b) n-BuLi, (CH2O)n, THF; (c) Red-Al, 0 C, then I2, THF 40 C; (d) DHP, PPTS, DCM, rt; (e) n-BuLi, (CH2O)n, THF, 78 C to 0 C; (f) TBDPSCl, imidazole, DMF, rt; (g) PPTS, EtOH. 28.6% over 7 steps; (h) L-(+)-DET, Ti(Oi-Pr)4, TBHP, DCM, 97%, >95% ee; (i) Terminal alkyne 7 in Scheme 2, Cp2ZrClH, MeMgCl, CuI, THF, 20 C, 91% yield⁄ ; (j) 2,2-dimethoxypropane, PPTS, DCM, 85% yield⁄ ; (k) TBAF, AcOH, THF, 89% yield⁄ ; (l) oxalyl chloride, DMSO, triethylamine, DCM, 78 C; (m) NaClO2, NaH2PO4, 2-methyl-2-butene, t-BuOH-H2O; (n) N,N-dimethylformamide di-tert-butyl acetal, 58% yield in 3 steps⁄ ; (o) 80% AcOH, THF, rt, 90% yield⁄ ; (p) Jones reagent, aqueous acetone, 10 C, 80% yield⁄ ; (q) the corresponding amine, HATU, Hunig base, 85% yield⁄ ; (r) TFA, anisole, DCM, 90% yield⁄ ; (s) H2-Pd/C, EtOH, 80%; (t) NaBH4, THF, MeOH, 93% yield. ⁄ yields when n = 5 and R1 = n-C7H15.
Paper
Development of a Kilogram-Scale Synthesis of a Novel Anti-HCV Agent, CH4930808
CH4630808 corrected
Abstract

Herein, we report the kilogram-scale synthesis of CH4930808 (1) CH 4630808 CORRECTED, a novel anti-hepatitis C virus agent. While pursuing improved productivity using many through-process strategies, we conducted scrupulous impurity control. Finally, we successfully developed a practical and scalable process for the synthesis of (1·1.5Na·2.5H2O), by which we prepared 3.28 kg of the active pharmaceutical ingredient for clinical studies
https://pubs.acs.org/doi/suppl/10.1021/acs.oprd.7b00383/suppl_file/op7b00383_si_001.pdf
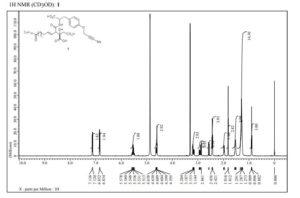
1H-NMR and 13C-NMR spectra of compound 5·HCl S 3– S 4
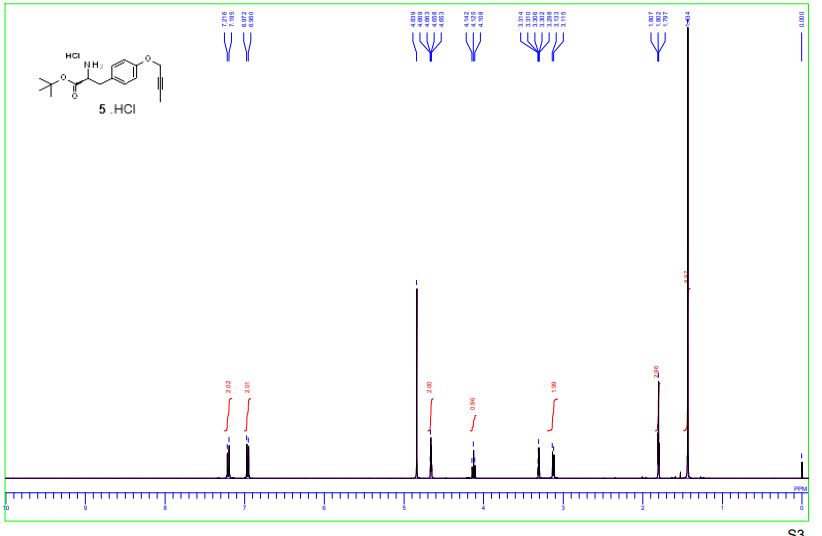

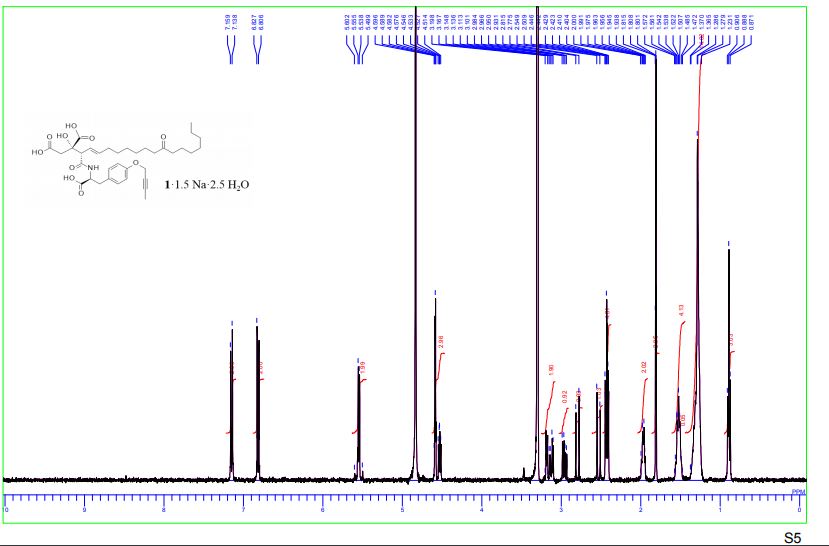
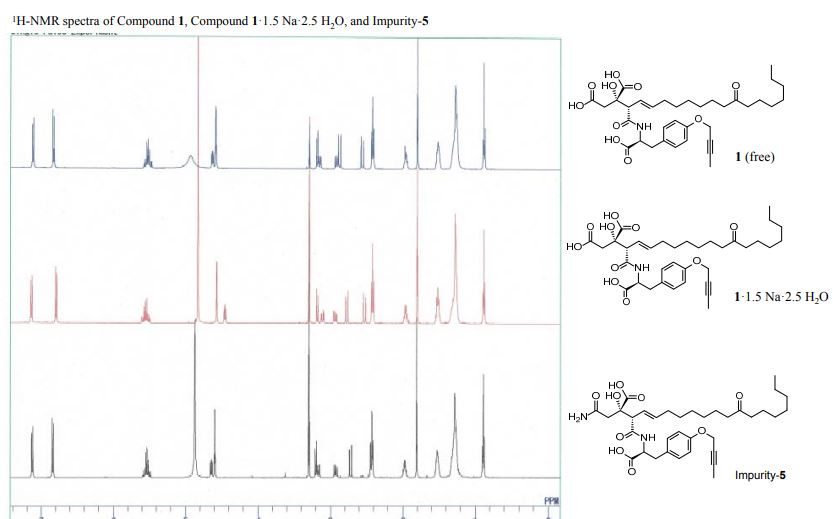
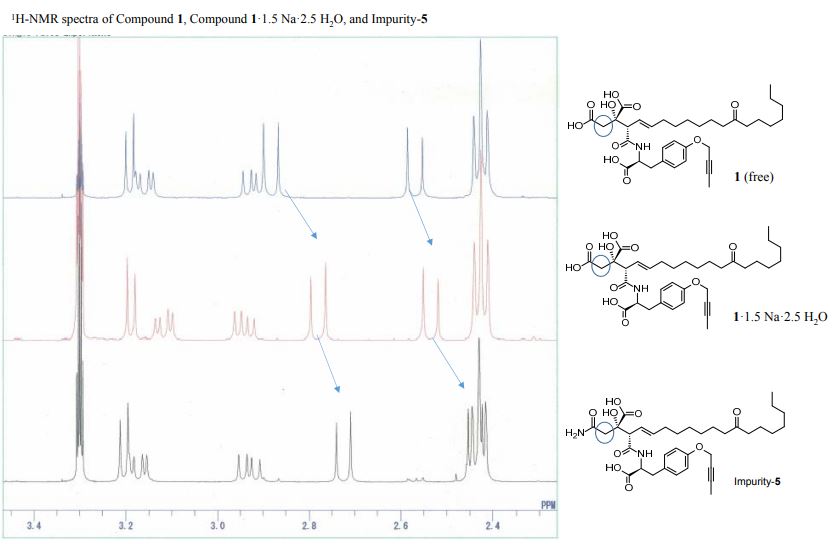
1H-NMR spectra of compound 1·1.5 Na·2.5 H2O S 5
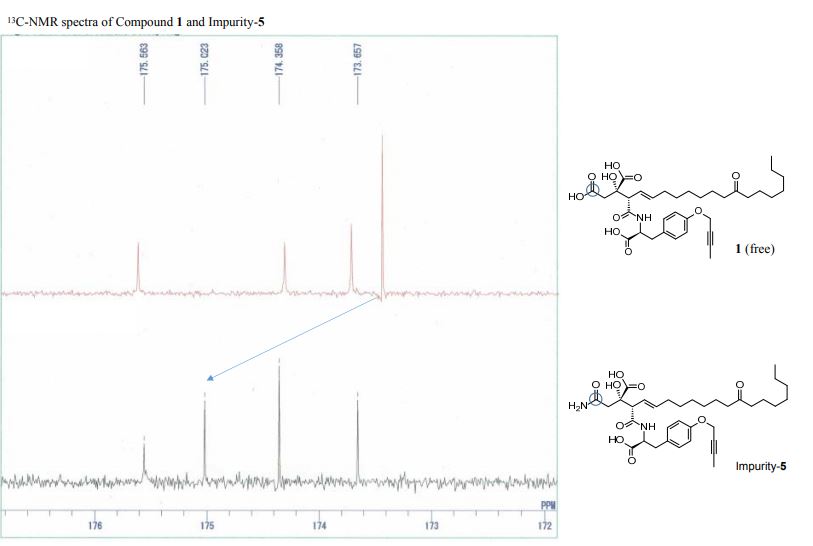
13C-NMR spectra compound 1·1.5 Na·2.5 H2O S 6
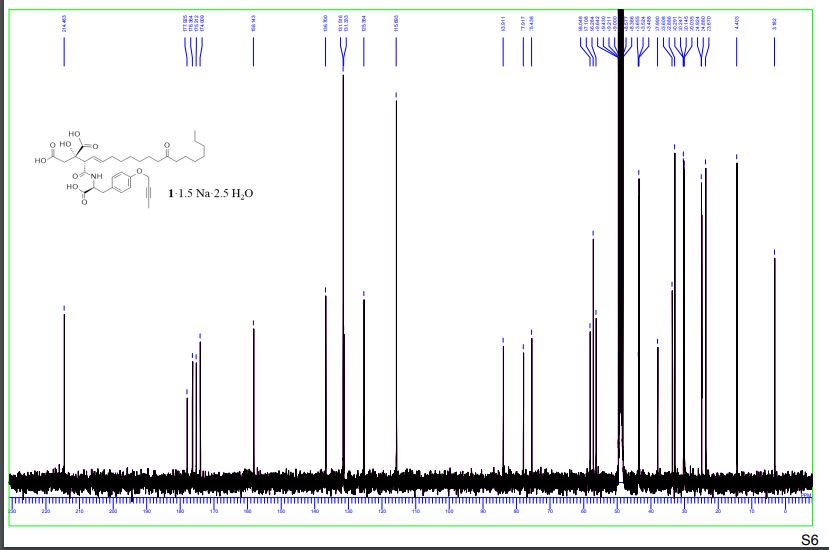

1H-COSY spectra of compound 1·1.5 Na·2.5 H2O S 7 – S 8


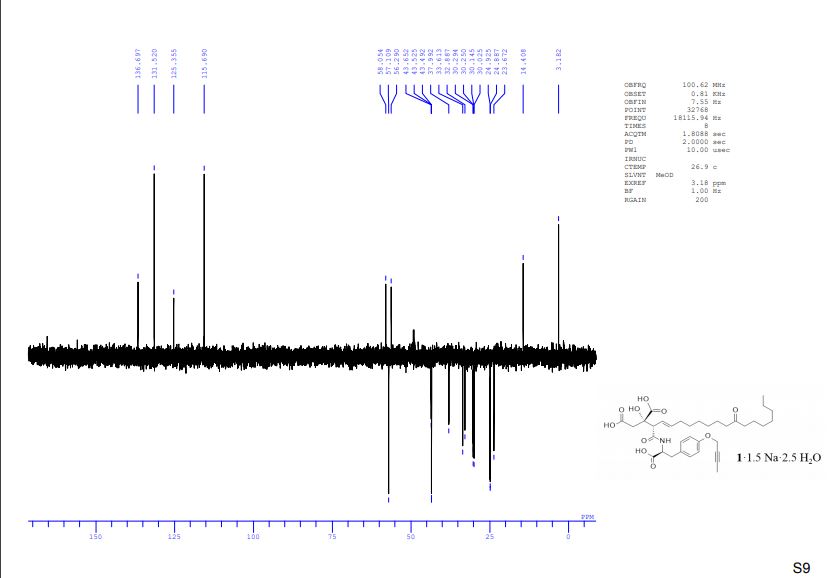
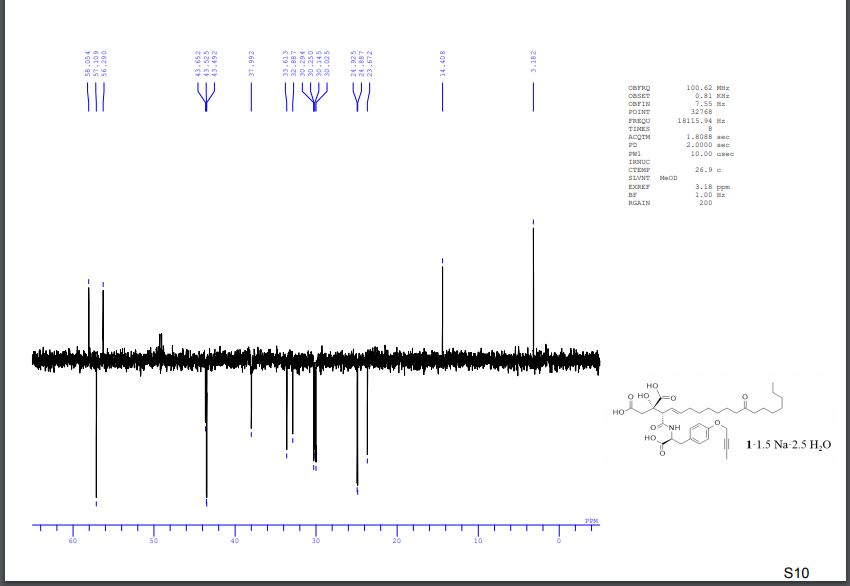
DEPT spectra of compound 1·1.5 Na·2.5 H2O S 9 – S 10
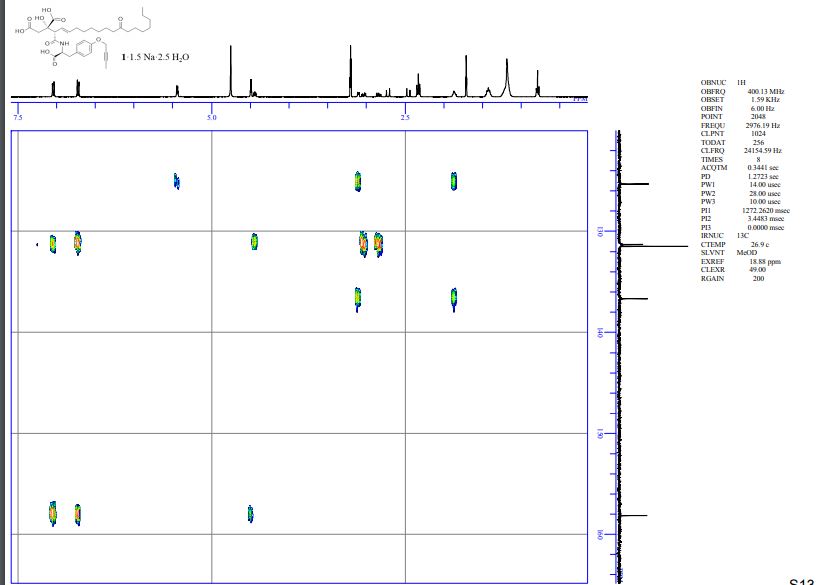
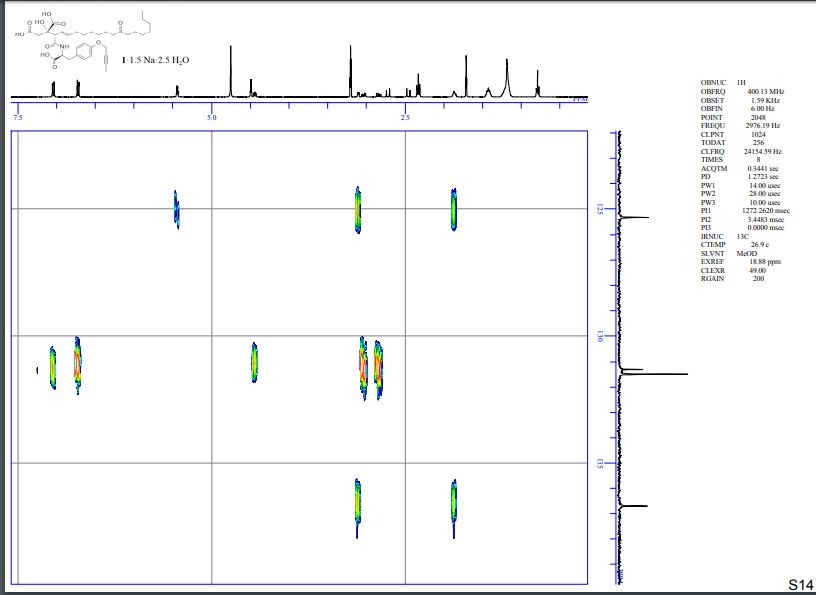
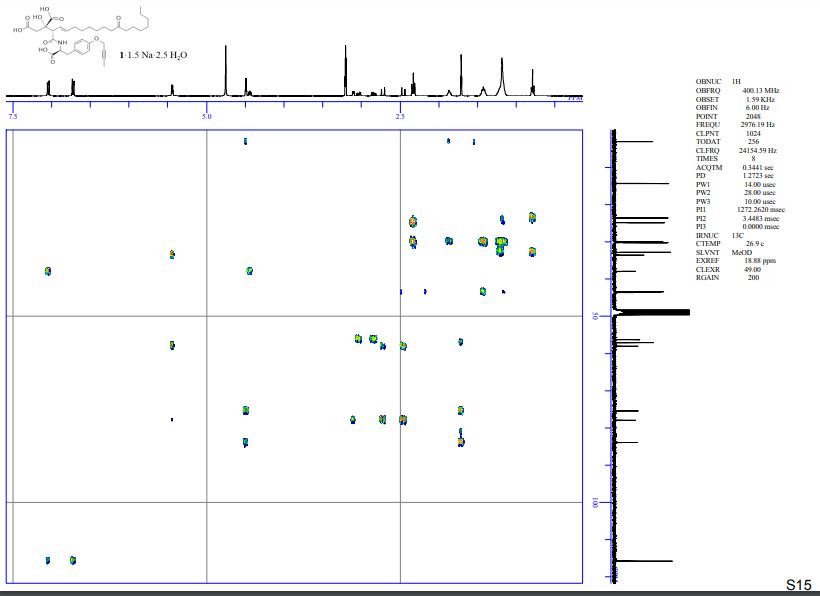
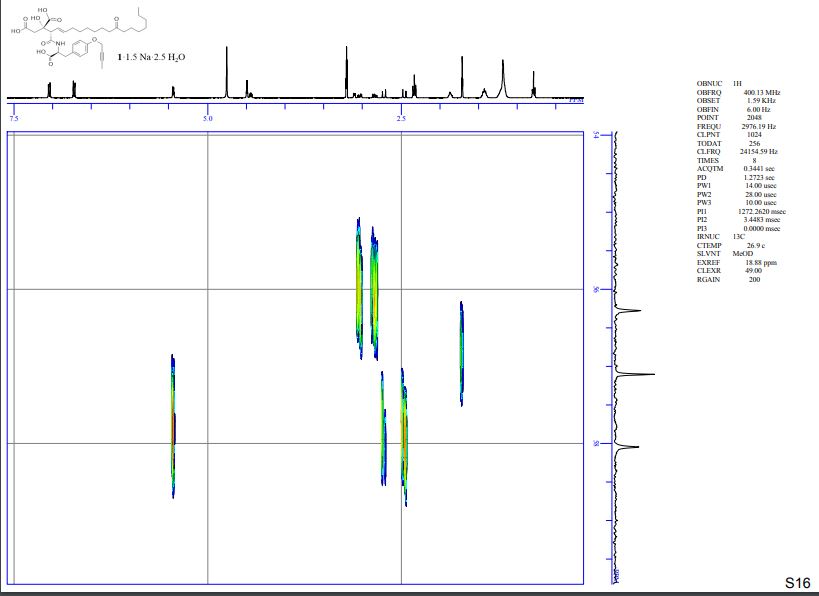
HMBC spectra of compound 1·1.5 Na·2.5 H2O S 11 – S 17

MASS
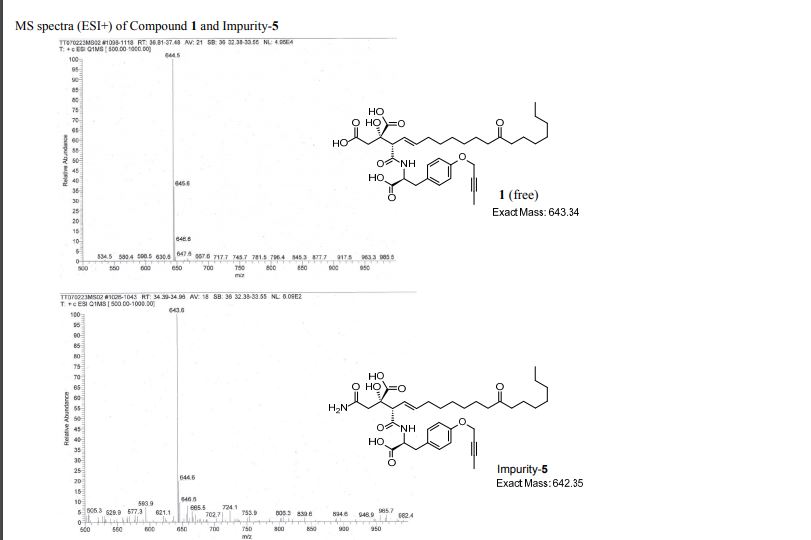
PATENT
WO 2004071503
WO 2005005372
WO 2006016657
WO 2006088071
WO 2007000994
WO 2007132882
WO 2009154248
WO 2014027696
PAPER
Angewandte Chemie, International Edition (2012), 51(17), 4218-4222, S4218/1-S4218/77.
Bioorganic & Medicinal Chemistry Letters (2013), 23(1), 336-339
PAPER
Organic & Biomolecular Chemistry (2017), 15(31), 6632-6639.
http://pubs.rsc.org/en/Content/ArticleLanding/2017/OB/C7OB01608E#!divAbstract
10.1039/C7OB01608E
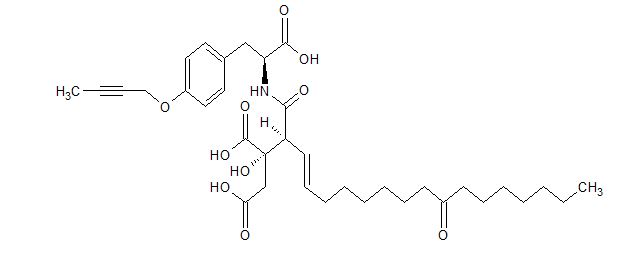
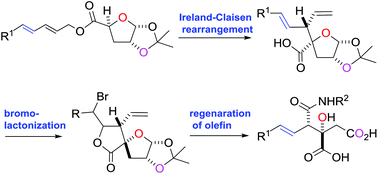
Stereoselective synthesis of the viridiofungin analogue NA808 from a chiral tetrahydrofuran-carboxylic acid
Abstract
The viridiofungin analogue NA808 was synthesized by the stereoselective Ireland–Claisen rearrangement of dienylmethyl ester, regioselective bromolactonization of β-divinylpropanoic acid and retro-bromolactonization.

The number of people infected with hepatitis C virus (HCV) is estimated at 1 to 200 million people worldwide, and over 2 million people in Japan. Approximately 50% of these patients migrate to chronic hepatitis, of which approximately 20% become liver cirrhosis, liver cancer after more than 30 years after infection. About 90% of liver cancer is said to be hepatitis C cause. In Japan, more than 20,000 patients die every year from liver cancer associated with HCV infection.
HCV was discovered in 1989 as a major causative virus of non-A non-B hepatitis after transfusion. HCV is an enveloped RNA virus whose genome
It consists of single-stranded (+) RNA and is classified as a genus Hepacivirus of Flaviviridae.
Since HCV avoids the immune mechanism of the host due to a cause which is still unclear, persistent infection is often established even when infected with an adult with developed immune mechanism, progresses to chronic hepatitis, liver cirrhosis, hepatocellular carcinoma, surgery It is also known that many patients have liver cancer recurrence due to inflammation that continues to occur in non-cancerous areas.
Therefore, establishment of an effective therapy for hepatitis C is desired, and among them, apart from coping therapy that suppresses inflammation by anti-inflammatory agents, development of a drug that reduces or eradicates HCV in the affected liver It is strongly desired.
Interferon treatment is currently known as the only effective treatment for HCV elimination. However, the number of patients with interferon effective is about one third of all patients. In particular, interferon response to HCV genotype 1 b is very low. Therefore, development of anti-HCV drugs that can replace or be used in combination with interferon is strongly desired.
In recent years, Ribavirin (1 – 3 – D – lipofuranosyl – 1 H – 1, 2, 4 – triazole – 3 – carboxamide) is commercially available as a therapeutic agent for hepatitis C by combining with interferon, Is still low, further new treatment for hepatitis C is desired. In addition, attempts have been made to eliminate viruses by enhancing the immune system of patients, such as interferon agonists, interleukin-12 agonists, etc. However, no effective drug has yet been found.
Since the HCV gene has been cloned, molecular biological analysis of the mechanism and function of viral genes, functions of proteins of each virus and the like has been accompanied by rapid development of forces, replication of virus in host cells, persistent infection, pathogenesis The mechanism such as sexuality has not been sufficiently elucidated, and at the present time, an HCV infection experiment system using reliable cultured cells has not been constructed. Conventionally, when evaluating anti-HCV drugs, alternative alternative virus method using other closely related viruses had to be used.
In recent years, however, it became possible to observe in vitro HCV replication using the nonstructural region part of HCV, so that anti-HCV drugs could be easily evaluated by the replicon assay method (Non-Patent Document 1). The mechanism of H CV RN A replication in this system is believed to be identical to the replication of the full-length HCV RNA genome infected with hepatocytes. Therefore, this system can be said to be a cell-based approach system useful for identifying compounds that inhibit the replication of HCV.
The compounds claimed in this patent are compounds that inhibit the replication of HCV found by the replicon astrocyte method. These inhibitors are considered highly likely to be therapeutic agents for HCV.
Non-Patent Document 1
B. Roman et al., Science (Science), 1999, 285, 110 – 113
Example 14

– 1 (Step 1 1)

According to the method described in the literature (J. Org. Chem. 1989, 45, 5522, BE Marron, et al)
TBDPSO.
a on
Of compound a (7.0 1 g) was synthesized, and anhydrous ethyl ether of this compound a
(700 ml) was cooled to 0 ° C. and bis (2-methoxyethoxy) aluminum hydride (414 mmol, 218 ml, 70% toluene solution) was added slowly. Five minutes after adding the reagent, the ice bath was removed and stirring was continued for 1 hour at room temperature. The reaction solution was cooled to 0 ° C and anhydrous ethyl acetate (1 9.8 ml, 203 mmol) was added slowly. After stirring at the same temperature for 10 minutes, it was cooled to 1 78 ° C., and iodine (76.1 g,
300 thigh 0 1) was added. The temperature was gradually raised to room temperature over 2 hours to complete the reaction. To the reaction solution was added aqueous sodium bisulfite solution, and ethyl acetate was added. The reaction solution was filtered with suction through celite, the organic layer was separated, and the aqueous layer was extracted again with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude title compound (100 g) as a light brown oily substance. The obtained crude product was directly used for the next reaction.
Physicochemical properties of compound b
Molecular weight 466
FAB-MS (positive mode, matrix m-NBA) 467 (M + H + ).
Chemical shift value of X H-NMR (in heavy chloroform) δ:
J = 6 Hz), 3.80 (2H, t, J = 6 Hz), 4.18 (2H, t, J = 5 Hz), 2.73 (2H, t, J = 6 Hz), 1.49 Hz, 5.91 (1 H, t, J = 5 Hz), 7.35 – 7.46 (6 H, m), 7.65 – 7.69 (4 H, m)
1 -2 (Step 1 – 2)
TBDPS

Dichloro port methane solution of compound b obtained in the above reaction (300 ml) was cooled to 0 ° C, dihydropyran (22. 7 ml, 248删0 plus 1). Pyridinium paratoluenesulfonic acid (260 mg, 1 mol) was added to this solution. After 1 hour sodium bicarbonate water was added to stop the reaction. The separated organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude compound c (108 g) thus obtained was directly used for the next reaction.
Physicochemical properties of compound c
Molecular weight 550
FAB-MS (positive mode, matrix m-NBA) 551 (M + H + )
Chemical shift value of 1 H – NMR (in heavy mouth formium) δ:
3.46-3.58 (2H, m), 3.76 (2H, t, J = 6 Hz), 3.82 (2H, t, J = 6 Hz), 1.04 (9H, s), 1.49-1.91 J = 13, 6 Hz), 4.65 (1 H, t, J = 3 Hz), 5.91 (1 H, t (s)), 4.93 (1 H, m), 4.06 (1 H, dd, J = 13, 6 Hz) , J = 5 Hz) 7.35 – 7.43 (6 H, m), 7.65 – 7.69 (4 H, m)
1-3 (Step 1- 3)
![]()
The crude compound c (4. 73 g) was dissolved in anhydrous ethyl ether (30 ml) and cooled to 1 78 ° C. Tert-butyllithium (1 7. 2 mol, 1 0.7 ml, 1.6 N pentane solution) was added slowly. After stirring at the same temperature for 1 hour, paraformaldehyde (1 8.9 mraol, 570 mg) was added and the mixture was warmed to 0 ° C. for 30 minutes at the same temperature and stirred for 1 hour. An aqueous solution of salthyanmonium was added to stop the reaction, and the mixture was extracted with ethyl acetate. The aqueous layer was extracted with a small amount of ethyl acetate and the combined organic layer was washed with saturated brine and dried over anhydrous sodium sulfate. The crude product obtained by concentration under reduced pressure was purified by column chromatography (silica gel, hexane-ethyl acetate 9: 1 to 4: 1) to give compound d (1. 635 g) as a colorless oily substance.
Physicochemical properties of compound d
Molecular weight 454
FAB-MS (positive mode, matrix m-NBA) 455 (M + H + )
^ – NMR (chemical shift value in heavy chloroform) δ:
J = 6 Hz), 3.03 (1 H, t, J = 6 Hz), 3.47 – 3.58 (2 H, m), 3.75 – 3.92 (2 H, (3 H, m), 4.08 – 4.26 (4 H, m), 4.68 (1 H, t, 3 Hz),
5.53 (1 H, t, J = 7 Hz) 7.35 – 7.47 (6 H, m), 7.64 – 7.68 (4 H, m)
1 -4 (Step 1 – 4)
![]()
An anhydrous N, N-dimethylformamide solution (2 ml) of the compound d (34 mg, 0. 76 mmol) and imidazole (71 mg, 1.14 mmol) was cooled to 0 ° C and tert- Chlorosilane (0: 2 ml, 0. 76 mmol) was capped and stirred for 2 hours. An ammonium chloride aqueous solution was added to stop the reaction, and the mixture was extracted with hexane. The organic layer was washed with water twice, followed by saturated brine and dried over anhydrous sodium sulfate. And concentrated under reduced pressure to obtain crude compound e (554 mg) as a colorless oily substance.
Physicochemical properties of compound e
FAB-MS (positive mode, matrix m-NBA) 715 (M + Na + )
Chemical shift value of ‘1 H-NMR (in heavy mouth formium) δ:
(4H, m), 1.00 J = 7 Hz), 5.43 (1 H, t, J = 7 Hz), 7.29 – 7.48 (12 H, m), 4.00 – 4.09 (1 H, m), 4.14 , 7.57 – 7.78 (8 H, m)
1-5 (Step 1- 5)
![]()
f
Pyridinium paratoluenesulfonic acid (9 O mg, 0.36 mmol) was added to an ethanol solution (6 ml) of the compound e (1. 16 g, 1. 67 mmol), and the mixture was stirred at 60 ° C. for 3.5 hours. After cooling the solution to room temperature, a saturated aqueous sodium bicarbonate solution was added and the mixture was extracted with ethyl acetate. The organic layer was washed successively with water and saturated brine, and dried over anhydrous sodium sulfate. The mixture was concentrated under reduced pressure, and the resulting crude product was purified by column chromatography (silica gel, hexane / ethyl acetate 20: 1) to give compound f (825 mg, 81%) as a colorless oily substance.
Physicochemical properties of compound f
Molecular weight 608
FAB-MS (positive mode, matrix m-NBA) 631 (M + Na + )
^ – NMR (chemical shift value in heavy chloroform) δ:
(2H, t, J = 7 Hz), 3.75 (2H, t, J = 7 Hz), 3.90 (2H, t, J = 7 Hz), 1.01 (9H, s), 1.01 , 7.59-7.47 (12 H m), 7.57-7.75 (8 H, m), 4.14 (2 H, s), 5 47 (1 H, t, J =
1-6 (Step 1-6)
![]()
9
The round bottom flask containing the rotor was heated and dried under reduced pressure and then purged with nitrogen, and anhydrous
Dichloromethane (60 ml) was added and cooled to _20 ° C. Titanium tetraisopropoxide (2.3 3 ml, 7.8 8 mmol), L 1 (+) – Jetyl tartrate (1.6 2 ml, 9. 4 6 min. 0 1) was added successively, and after stirring for 15 minutes, compound f (4.80 g, 7. 88 mmol) in dichloromethane (30 ml), and the mixture was stirred for 15 minutes. Cool to _ 25 ° C and add tert-butyl hydroperoxide (5. 25 ml,
15. 8 mmol, 3 N dichloromethane solution) was slowly added dropwise. After completion of the dropwise addition, the mixture was stirred at 20 ° C. for 2 hours, dimethylsulfide (1.1 ml) was added, and the mixture was further stirred at the same temperature for 1 hour. A 10% aqueous solution of tartaric acid was added to the reaction solution and the mixture was stirred for 30 minutes, and then stirred at room temperature for 1 hour. The organic layer was separated, the aqueous layer was extracted with a small amount of dichloromethane and the combined organic layers were dried over anhydrous sodium sulfate. The crude product obtained was concentrated under reduced pressure, and purified by force RAM chromatography (silica gel, hexane / monoacetic acid ethyl 9: 1). Compound g (4. 78 g, 97%) was obtained as a colorless oily substance. The asymmetric yield (> 95% ee) was determined by NMR analysis of the corresponding MT PA ester.
Physicochemical properties of compound g
Molecular weight 624
F AB-MS (positive mode, matrix m-NBA) 647 (M + Na + )
– Chemical shift value of NMR (in heavy chloroform) δ:
J = 14, 7 Hz), 2.23 (1 H, dt, J = 14, 1 H), 1.02 (9 H, s), 1.03 (9 H, s), 1.72 (6H, m), 7.32-7.45 (12H, m), 7.60- 7.65 (8H, m), 6.5 Hz), 3.17 (1H, dd, J = 6, 5 Hz), 3.55-3.79
1 – 7 (Step 1 – 7)

To a solution (100 ml) of the compound α (10. 45 g, 37.2 mmol) produced in the step 2-3 of Production Example 1 described below in an anhydrous tetrahydrofuran solution (100 ml) under a nitrogen atmosphere was added biscyclopentadienylzirconium hydride chloride (10. lg, 37.2 mol) was added at room temperature and stirred for 30 minutes. The resulting solution was cooled to 1780C and methyl magnesium chloride (24.7 ml, 74 mmol, 3 N tetrahydrofuran
Furan solution), and the mixture was stirred for 5 minutes. Monovalent copper iodide (500 mg, 7.2 mM) was added to this solution and the temperature was gradually raised to _ 30 ° C. An anhydrous tetrahydrofuran solution (70 ml) of the compound g (4. 49 g) was added over 20 minutes, and after completion of the dropwise addition, the mixture was stirred at 25 ° C. overnight. The saturated ammonium chloride aqueous solution was slowly added, the reaction was stopped, and the temperature was gradually raised to room temperature. The mixture was stirred at room temperature for 10 hours and the resulting white solid was filtered off through celite. The celite was washed thoroughly with ethyl acetate and the organic layer was separated. The aqueous layer was extracted with a small amount of ethyl acetate and the combined organic layer was washed with saturated aqueous ammonium chloride solution and then dried over anhydrous sodium sulfate. Concentrated under reduced pressure and the obtained crude product was purified by column chromatography (silica gel, hexyl acetate
20: 1 to 9: 1) to give compound h (5. 96 g, 91%) as a pale yellow oily substance.
Physicochemical properties of compound h
Molecular weight 907
F AB – MS (negative mode, matrix πι – Α Β A) 906 (Μ – Η + )
Chemical shift value of 1 H-NMR (in heavy chloroform) δ:
0.88 (3H, t, ![]()
0.99 (9H, s), 1.04 (9H, s), 1.18-1.63 (22H, m), 1.78-2.01 (4H, m), 2.44-2.57 (1H, m), 3.00 (1H, t, J = 6 Hz), 3.59-3.92 (10H, m), 4.28 (1H, s), 5.37-5.55 (2H, m), 7.29-7.65 (20H, m)
1-8 (Step 1-8)

Compound h (5.30 g, 5.84 dragon ol) was dissolved in dichloromethane (200 ml) and 2, 2-dimethoxypropane (150 ml), pyridinium paratoluenesulfonic acid (15 mg, 0.058 mmol) was added , And the mixture was stirred at room temperature overnight. The reaction was quenched by adding saturated aqueous sodium bicarbonate and extracted twice with dichloromethane. After drying over anhydrous sodium sulfate, the mixture was concentrated under reduced pressure, and the resulting crude product was purified by column chromatography (silica gel, hexane-ethyl acetate 20: 1). Compound i (4. 69 g, 86%) was obtained as a pale yellow oily substance.
Physicochemical properties of Compound i
Molecular weight 947
F AB-MS (negative mode, matrix m-NBA) 946 (M – H + )
Chemical shift value of 1 H – NMR (in heavy mouth formium) δ:
(1 H, m), 0.88 (3H, t, J = 6 Hz), 1.02 (9H, s), 1.05 (9H, s), 1.14-1.63 (28H, m), 1. 2.16 (2H, m), 7.28 – 7.47 (12H, m), 7.61 – 7.69 (1H, d, J = 10 Hz), 3.64-3.86 (6H, m 3.92 (s, 4H), 5.36-5.42 8 H, m) 1 – 9 (Step 1 – 9)

A tetrahydrofuran solution (50 ml) of the compound i (4. 39 g, 4. 64 mmol) was cooled to 0 ° C., tetrabutylammonium fluoride (10. 2 ml, 10, 2 difficulty, 1 M tetrahydrofuran solution) and Acetic acid (0. 53 ml, 9. 27 mmol) was added. The temperature was gradually raised to room temperature and stirred for 2 days. A saturated ammonium chloride aqueous solution was added and the mixture was extracted twice with dichloromethane. The combined organic layer was washed with aqueous sodium bicarbonate and dried over anhydrous sodium sulfate. The crude product was purified by column chromatography (silica gel, hexane-ethyl acetate 9: 1 to 3: 2) to obtain the compound〗 (1. 73 g, 81%) Was obtained as a pale yellow oily substance.
Physicochemical properties of compound j
Molecular weight 470
F AB-MS (positive mode, matrix m-NBA) 493 (M + Na + )
Chemical shift value of X H-NMR (in heavy chloroform) δ:
2.73 (1H, dt, J = 6, 10 Hz), 2.95 (3H, t, J = 6 Hz), 1.17-1.73 (26H, m), 1.91-2.16 (4H, m), 2.4 J = 15, 7 Hz (1 H, dt, J = 15 Hz), 3.48 (1 H, d, J = 1 Hz), 3.63-4.01 (m, 10 H), 5.15 )
1-10 (Step 1- 10)

Under an atmosphere of nitrogen, a solution of oxalyl chloride (0. 575 ml, 6. 6 mol) in anhydrous dichloromethane (17 ml) was cooled to 178 ° C. and dimethyl sulfoxide
(0. 9 36 ml, 1 3 2 minol) in dichloromethane (1 ml) was added dropwise and the mixture was stirred for 15 minutes. Dichloromethane solution (5 ml) of compound j (388 mg, 0. 824 aura) was slowly added dropwise. The mixture was stirred at the same temperature for 1 hour, then terethylamine (3 ml, 21.4fflmol) was added and the mixture was stirred for 30 minutes. The cooling bath was removed and a low-boiling compound was removed by blowing a nitrogen gas stream to the solution, followed by drying under reduced pressure. Jether ether (15 ml) was added to the residue, and insoluble matter was filtered off and concentrated. After this operation was carried out twice, the obtained residue was immediately used for the next reaction.
The crude dialdehyde was dissolved in 2-methyl-2-propanol (24 ml) and 2-methyl-2-butene (6 ml) and cooled to about 5 to 7 ° C. To this solution was added sodium chlorite (745 mg, 8. 24 mmol) and sodium dihydrogenphosphate
(745 mg, 6. 2 l mmol) in water (7. 45 ml) was slowly added dropwise. After 2 hours the mixture was cooled to 0 ° C. and aqueous sodium hydrogenphosphate solution was added to adjust PH to approximately 5. The mixture was extracted three times with dichloromethane, and the combined organic layer was washed with saturated brine and then dried over anhydrous sodium sulfate. After filtration, concentration under reduced pressure afforded a pale yellow oily residue which was immediately used for the next reaction without further purification.
The crude dicarboxylic acid was dissolved in N, N-dimethylformamide di tert-butylacetal (4. 5 ml) and stirred at 70 ° C. for 1 hour. The low boiling point compound was distilled off under reduced pressure. The residue was purified by column chromatography (silica gel, hexane / ethyl acetate 20: 1) to give compound k (340 mg, 60%) as a pale yellow oily substance.
Physicochemical properties of compound k
Molecular weight 6 10
FAB-MS (positive mode, matrix m-NBA) (M + H + ) 611, (M + Na + ) 633
^ – NMR (chemical shift value in heavy chloroform) δ:
(2H, ABq, J = 15, 18 Hz), 2.93 (1 H, q, J = 6 Hz), 1.18 J = 7 Hz), 3.82-3.88 (2H, m), 3.92 (4H, s), 5.51-5.69 (2H, m)
1- 11 (Step 1 – 1 1)

Compound k (34 mg, 0. 556 mmol) was dissolved in tetrahydrofuran (1 ml), 80% acetic acid aqueous solution (10 ml) was added, and the mixture was stirred at room temperature for 3.5 hours. The mixture was slowly added into a saturated aqueous solution of sodium bicarbonate to neutralize acetic acid and then extracted twice with ethyl acetate. Drying over anhydrous sodium sulfate, followed by filtration and concentration under reduced pressure to give compound t
(290 mg, 99%) as a pale yellow oil.
Physicochemical properties of compound f
Molecular weight 526
FAB – MS (positive mode, matrix m – NBA) (M + H + ) 527,
(M + Na + ) 549
Chemical shift value of iH-NMR (in heavy chloroform) δ:
(2H, Q ![]()
, 2.25-2.41 (5H, m), 1.99 (1H, d, J = 7 Hz), 2.04 (1H, d (1H, t, 7 Hz), 1.18- 1.68 (36H, ra), 2.01 J = 7 Hz), 5.58 (1 H, dt, J = 16, 6 Hz), 3.62 (3H, m), 3.99 (1H, s), 5.42
1-12 (Step 1 – 12)

Acetone (45 ml) was cooled to 0 ° C. and Jyones reagent (0.48 ml, 0.9 mmol, 1.8 9 N) was added. An acetone solution (3 ml) of the compound (216 mg, 0, 41) was slowly added dropwise to this mixture. Stirring at the same temperature for 1 hour
After stirring, the reaction was stopped by adding an aqueous sodium bisulfite solution until the yellow color of the reaction disappeared and a dark green precipitate appeared. A saturated saline solution (20 ml) was added thereto, and the mixture was extracted twice with dichloromethane, and the combined organic layer was dried over anhydrous sodium sulfate. The mixture was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (dichloromethane monomethanol 50: 1 to 20: 1) to give compound m (198 mg, 89%) as a pale yellow oily substance.
Physicochemical properties of compound m
Molecular weight 541
ESI (L CZMS positive mode) (M + H + ) 542
Chemical shift value of 1 H – NMR (in heavy mouth formium) δ:
J = 8 Hz), 2.70 (1 H, t, J = 6 Hz), 1.16 – 1.67 (36 H, m), 1.99 (2 H, J = 15, 5 Hz), 2.68 (1 H, d, J = 9 Hz), 3.28 )
1 – 13 (Step 1 – 13)

A solution of the compound m (6. 4 mg, 0.12 mmol), a solution of (S) -4- (2-butynyloxy) phenylalanine t-butyl ester hydrochloride (4.6 mg, 0.114 mmol) in N, N-dimethylformamide lml) was cooled to 110 ° C and N, N-diisopropylethylamine (0 ° 5 ml, 0.026 mmol), O- (7-azobenzotriazole 1- 1, N, N, N ‘, N’ – tetramethyluronium hexafluorophosphate (7.0 mg, 0.17 mmol) was added sequentially. The temperature was raised to room temperature with stirring and stirred overnight. An aqueous ammonium chloride solution was added to terminate the reaction, and the mixture was extracted with ethyl acetate. The organic layer was washed twice with water and then with saturated brine, and then dried over anhydrous sodium sulfate. After filtration and concentration under reduced pressure, the residue was purified by thin layer silica gel thin layer chromatography (hexane / ethyl acetate 7: 3) to obtain compound n
(8. 4 mg, 88%) as a colorless solid.
Physicochemical properties of compound n
![]()
^ – NMR (chemical shift value in heavy chloroform) δ:
J = 1.9 Hz), 1.90-2.03 (2H, m), 2.29 – 2.43 (4H, t, J = 6.9 Hz), 1 .12-1.68 (45H, m), 1.85 (3H, m), 4.22 (1 H, s), 4.57 – 4.74 (3H, d, J = 16.5 Hz) J = 8.6 Hz), 7.01 (1 H, d, J = 8.6 Hz), 5.46 (1 H, dd J = 9.2, 15.2 Hz), 5.64 (1 H, dt, J = 6.6, 15.2 Hz) 7.9 Hz), 7.13 (2H, d, J – 8.6 Hz)
1-14 (Step 1 – 14)

Dichloromethane solution (3 ml) of compound n (8.4 mg) was cooled to 0 ° C and anisanol (0.01 ml) and trifluoroacetic acid (1 ml) were sequentially added. Slowly warmed to room temperature and stirred overnight. After concentrating the reaction solution under reduced pressure and azeotropically twice with benzene, the residue was purified with megabond-1-butanediol (500 mg, Parian) (dichloromethane-methanol = 20: 1) to obtain Compound 21 (5. 3 mg, 80% As a colorless solid.
Physicochemical properties of compound 21 ‘

Molecular weight 643
ESI (LC / MS positive mode) 644 (M + H +)
Chemical shift value of 1 H – NMR (in methanol d – 4) δ:
0.90 (3 H, t, J = 7 Hz), 1.19 – 1.38 (1 m), 1.42 – 1.60 (cm), 1.82 (3 H, t,
J = 2 Hz), 2.8 – 2.98 (2 H, m), 3.09 – 3.23 (2 H, m), 2.8 (2H, d, J = 9 Hz) 7 7.13 (2H, d, J = 9 Hz), 4.53 – 4.67 (3H, m), 5.39-5.61 (2H, m), 6.83
References
Discovery of NA808: A novel host targeting anti-HCV agent
237th Am Chem Soc (ACS) Natl Meet (March 22-26, Salt Lake City) 2009, Abst MEDI 14
///////////////CH4630808, CH 4630808, NA 808
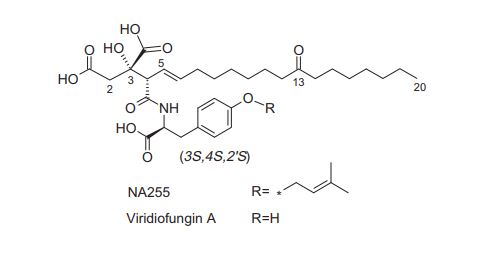
Viridiofungin A
CCCCCCCC(=O)CCCCCCC=CC(C(=O)NC(CC1=CC=C(C=C1)O)C(=O)O)C(CC(=O)O)(C(=O)O)O
TITLE COMPD
O=C(O)[C@](O)(CC(=O)O)[C@H](\C=C\CCCCCCC(=O)CCCCCCC)C(=O)N[C@@H](Cc1ccc(OCC#CC)cc1)C(=O)O