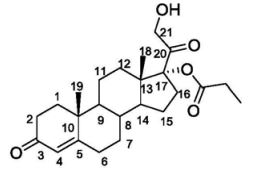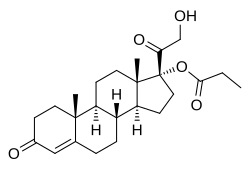
Clascoterone
(1R,3aS,3bR,9aR,9bS,11aS)-1-(2-hydroxyacetyl)-9a,11a-dimethyl-7-oxo-1H,2H,3H,3aH,3bH,4H,5H,7H,8H,9H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-1-yl propanoate
| Formula |
C24H34O5
|
|---|---|
| CAS |
19608-29-8
|
| Mol weight |
402.5238
|
FDA APPROVED, 2020/8/26, Winlevi
|
クラスコステロン;
|
Anti-acne, Androgen receptor antagonist
Clascoterone, sold under the brand name Winlevi, is an antiandrogen medication which is used topically in the treatment of acne.[1][2][3] It is also under development for the treatment of androgen-dependent scalp hair loss.[2] The medication is used as a cream by application to the skin, for instance the face and scalp.[3]
Clascoterone is an antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone.[4][5] It shows no systemic absorption when applied to skin.[3]
The medication, developed by Cassiopea and Intrepid Therapeutics,[2] was approved by the US Food and Drug Administration (FDA) for acne in August 2020.[6][7]
Medical uses
Clascoterone is indicated for the topical treatment of acne vulgaris in females and males age 12 years and older.[1][8] It is applied to the affected skin area in a dose of 1 mg cream (or 10 mg clascoterone) twice per day, once in the morning and once in the evening.[1] The medication should not be used ophthalmically, orally, or vaginally.[1]
Available forms
Clascoterone is available in the form of a 1% (10 mg/g) cream for topical use.[1]
Contraindications
Clascoterone has no contraindications.[1]
Side effects
The incidences of local skin reactions with clascoterone were similar to placebo in two large phase 3 randomized controlled trials.[1][9] Suppression of the hypothalamic–pituitary–adrenal axis (HPA axis) may occur during clascoterone therapy in some individuals due to its cortexolone metabolite.[1][8] HPA axis suppression as measured by the cosyntropin stimulation test was observed to occur in 3 of 42 (7%) of adolescents and adults using clascoterone for acne.[1][8] HPA axis function returned to normal within 4 weeks following discontinuation of clascoterone.[1][8] Hyperkalemia (elevated potassium levels) occurred in 5% of clascoterone-treated individuals and 4% of placebo-treated individuals.[1]
Pharmacology
Pharmacodynamics
Clascoterone is an steroidal antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone (DHT).[1][4][5] In a bioassay, the topical potency of the medication was greater than that of progesterone, flutamide, and finasteride and was equivalent to that of cyproterone acetate.[10] Likewise, it is significantly more efficacious as an antiandrogen than other AR antagonists such as enzalutamide and spironolactone in scalp dermal papilla cells and sebocytes in vitro.[5]\
Pharmacokinetics
Steady-state levels of clascoterone occur within 5 days of twice daily administration.[1] At a dosage of 6 g clascoterone cream applied twice daily, maximal circulating levels of clascoterone were 4.5 ± 2.9 ng/mL, area-under-the-curve levels over the dosing interval were 37.1 ± 22.3 h*ng/mL, and average circulating levels of clascoterone were 3.1 ± 1.9 ng/mL.[1] In rodents, clascoterone has been found to possess strong local antiandrogenic activity, but negligible systemic antiandrogenic activity when administered via subcutaneous injection.[10] Along these lines, the medication is not progonadotropic in animals.[10]
The plasma protein binding of clascoterone is 84 to 89% regardless of concentration.[1]
Clascoterone is rapidly hydrolyzed into cortexolone (11-deoxycortisol) and this compound is a possible primary metabolite of clascoterone based on in-vitro studies in human liver cells.[1][8] During treatment with clascoterone, cortexolone levels were detectable and generally below or near the low limit of quantification (0.5 ng/mL).[1] Clascoterone may also produce other metabolites, including conjugates.[1]
The elimination of clascoterone has not been fully characterized in humans.[1]
Chemistry
Clascoterone, also known as cortexolone 17α-propionate or 11-deoxycortisol 17α-propionate, as well as 17α,21-dihydroxyprogesterone 17α-propionate or 17α,21-dihydroxypregn-4-en-3,20-dione 17α-propionate, is a synthetic pregnane steroid and a derivative of progesterone and 11-deoxycortisol (cortexolone).[11] It is specifically the C17α propionate ester of 11-deoxycortisol.[10]
An analogue of clascoterone is 9,11-dehydrocortexolone 17α-butyrate (CB-03-04).[12]
History
C17α esters of 11-deoxycortisol were unexpectedly found to possess antiandrogenic activity.[10] Clascoterone, also known as cortexolone 17α-propionate, was selected for development based on its optimal drug profile.[10] The medication was approved by the US Food and Drug Administration (FDA) for the treatment of acne in August 2020.[6]
Two large phase 3 randomized controlled trials evaluated the effectiveness of clascoterone for the treatment of acne over a period of 12 weeks.[1][8][9] Clascoterone decreased acne symptoms by about 8 to 18% more than placebo.[1][9] The defined treatment success endpoint was achieved in about 18 to 20% of individuals with clascoterone relative to about 7 to 9% of individuals with placebo.[1][8][9] The comparative effectiveness of clascoterone between males and females was not described.[1][9]
A small pilot randomized controlled trial in 2011, found that clascoterone cream decreased acne symptoms to a similar or significantly greater extent than tretinoin 0.05% cream.[8][13] No active comparator was used in the phase III clinical trials of clascoterone for acne.[8] Hence, it’s unclear how clascoterone compares to other therapies used in the treatment of acne.[8]
The FDA approved clascoterone based on evidence from two clinical trials (Trial 1/NCT02608450 and Trial 2/NCT02608476) of 1440 participants 9 to 58 years of age with acne vulgaris.[14] The trials were conducted at 99 sites in the United States, Poland, Romania, Bulgaria, Ukraine, Georgia, and Serbia.[14]
Participants applied clascoterone or vehicle (placebo) cream twice daily for 12 weeks.[14] Neither the participants nor the health care providers knew which treatment was being given until after the trial was completed.[14] The benefit of clascoterone in comparison to placebo was assessed after 12 weeks of treatment using the Investigator’s Global Assessment (IGA) score that measures the severity of disease (on a scale from 0 to 4) and a decrease in the number of acne lesions.[14]
Society and culture
Names
Clascoterone is the generic name of the drug and its INN and USAN.[11][15]
Research
Clascoterone has been suggested as a possible treatment for hidradenitis suppurativa (acne inversa), an androgen-dependent skin condition.[16]
………………………………………………………………………….
PATENT
CN 112028956
https://patents.google.com/patent/CN112028956A/en
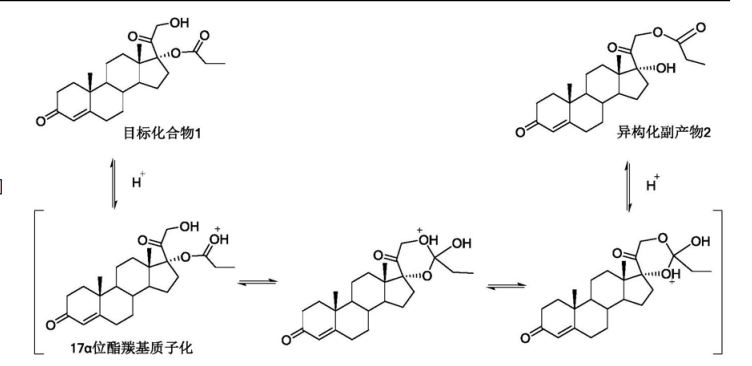
Abstract
Several 17α-monoesters of cortexolone and its Δ9-derivative are endowed with antiandrogenic activity. Their synthesis can be accomplished by means of a lipase-catalyzed chemoselective alcoholysis of the corresponding 17α,21-diesters.
Graphical abstract

1H NMR (500 MHz, CDCl3): selected data δ 5.78 (br s, 1H, H-4), 4.32 (dd, 1H, H-21, J18.3 and 4.9 Hz), 4.25 (dd, 1H, H-21, J18.3 and 4.9 Hz), 1.22 (s, 3H, CH3-19), 1.17 (t, 3H, CH3, J7.6 Hz), 0.72 (s, 3H, CH3-18) MP 133 °C (t-butylmethylether)
…………………………………………………………………..
PATENT
https://patents.google.com/patent/EP2503005B1/en
-
Cortexolone derivatives in which the hydroxyl group at position C-17α is esterified with short chain aliphatic or aromatic acids and the derivatives of the corresponding 9,11-dehydro derivative, are known to have an antiandrogenic effect.
- [0002]
EP 1421099 describes cortexolone 17α-propionate and 9,11-dehydro-cortexolone-17-α-butanoate regarding a high antiandrogenic biological activity demonstrated both “in vitro” and “in vivo” on the animal.
- [0003]
US3530038 discloses the preparation of a crystalline form of cortexolone-17α-propionate having a melting point of 126-129 °C and an IR spectrum with bands at (cm-1): 3500, 1732, 1713, 1655 and 1617.
- [0004]
A method for obtaining the above mentioned derivatives is described by Gardi et al. (Gazz. Chim. It. 63, 43 1,1963) and in the United States patent US3152154 providing for the transformation of cortexolone, or transformation of 9,11-dehydrocortexolone, in the intermediate orthoester using orthoesters available in the market as a mixture of aprotic solvents such as cyclohexane and DMF, in presence of acid catalysis (ex. PTSA.H20). The intermediate orthoester thus obtained can be used as is or upon purification by suspension in a solvent capable of solubilising impurities, preferably in alcohols. The subsequent hydrolysis in a hydroalcoholic solution, buffered to pH 4-5 preferably in acetate buffer, provides the desired monoester.
- [0005]
- [0006]
However, the monoesters thus obtained were, in the reaction conditions, unstable and, consequently hard to manipulate and isolate (R. Gardi et al Tetrahedron Letters, 448, 1961). The instability is above all due to the secondary reaction of migration of the esterifying acyl group from position 17 to position 21.
- [0007]
It is thus known that in order to obtain the above mentioned monoesters with a chemical purity in such a manner to be able to proceed to the biological tests, it is necessary to use, at the end of the synthesis, a purification process which is generally performed by means of column chromatography.
- [0008]
Furthermore, US3152154 describes how the hydrolysis of the diester in a basic environment is not convenient due to the formation of a mixture of 17α,21-diol, of 17- and 21 -monoesters, alongside the initial non-reacted product.
- [0009]
Now, it has been surprisingly discovered that an alcoholysis reaction using a lipase from Candida as a biocatalyst can be usefully applied during the preparation of 17α monoesters of cortexolone, or its 9,11-dehydroderivatives.
- [0010]
- [0011]
The chemoselectivity of the special enzymatic reaction in alcoholysis conditions, according to the present invention, opens new perspectives for preparation, at industrial level with higher yields, of 17α-monoesters with respect to the methods already indicated in literature.
- [0012]
The diesters serving as a substrate for the reaction of the invention can be prepared according to the prior art, for example following the one described in B.Turner, (Journal of American Chemical Society, 75, 3489, 1953) which provides for the esterification of corticosteroids with a linear carboxylic acid in presence of its anhydride and PTSA monohydrate.
EXAMPLES
- Example 1
Alcoholysis with CCL of cortexolone 17α, 21-dipropionate
- [0055]
Add butanol (0.4g, 5.45 mmoles) and CCL (17.4g, 3.86 U/mg, FLUKA) to a solution of cortexolone-17α,21-dipropionate (0.5g, 1.09 mmoles) in toluene (50ml). Maintain the mixture under stirring, at 30 °C, following the progress of the reaction in TLC (Toluene/ethyl acetate 6/4) until the initial material is dissolved (24h). Remove the enzyme by means of filtration using a Celite layer. Recover the cortexolone 17α-propionate (0.437, 99%) after evaporation under low pressure. Through crystallisation, from diisopropyl ether you obtain a product with a purity >99% in HPLC.
- [0056]
1H-NMR (500MHz, CDCl3) relevant signals δ (ppm) 5.78 (br s, 1 H, H-4), 4.32 (dd, 1 H, H-21), 4.25 (dd, 1H, H-21), 1.22 (s, 3H, CH3-19), 1.17 (t, 3H, CH3), 0.72 (s, 3H, CH3-18). P.f. 114 °C
Example 2 (comparative)
- [0057]
According to the method described in example 1 prepare cortexolone-17α-butanoate.
- [0058]
1H-NMR relevant signals δ (ppm) 5.78 (br s, 1H, H-4), 4.32 (dd, 1H, H-21), 4.26 (dd, 1H, H-21), 1.23 (s, 3H, CH3-19), 0.97 (t, 3H, CH3), 0.73 (s, 3H. CH3-18). P.F. 134-136 °C
Example 3 (comparative)
According to the method described in the example prepare cortexolone-17α-valerate.
- [0059]
1H-NMR relevant signals δ (ppm) 5.77 (br s, 1H, H-4), 4.32 (dd, 1H, H-21), 4.26 (dd, 1H, H-21), 1.22 (s, 3H, CH3-19), 0.95 (t, 3H, CH3), 0.72 (s, 3H, CH3-18). P.f. 114 °C (diisopropyl ether).
Example 4 (comparative)
According to the method described in the example prepare 9, 11-dehydro-cortexolone-17α-butanoate.
- [0060]
1H-NMR relevant signals δ (ppm) 5.77 (br s, 1H, H-4), 5.54 (m, 1H, H-9), 4.29 (dd, 1H, H-21), 4.24 (dd, 1H, H-21), 1.32 (s, 3H, CH3-19), 0.94(t, 3H, CH3), 0.68 (s, 3H, CH3-18). P.f. 135-136 °C (acetone/hexane).
Example 5
Alcoholysis with CALB of cartexolone-17α, 21-dipropionate
- [0061]
Dissolve cortexolone, 17α, 2-dipropionate (0.5g, 1.09 mmoles) in acetonitrile (40ml), add CALB (2.3g, 2.5 U/mg Fluka) and octanol (0.875ml). Leave the mixture under stirring, at 30 °C, for 76 hrs. Remove the enzyme by means of filtration using a paper filter. Once the solvents evaporate, recover a solid (0.4758) which upon analysis 1H-NMR shall appear made up of cortexolone-17α-propionate at 91%.
Example 6
Crystallisation
- [0062]
Add the solvent (t-butylmethylether or diisopropylether) to the sample according to the ratios indicated in Table 3. Heat the mixture to the boiling temperature of the solvent, under stirring, until the sample dissolves completely. Cool to room temperature and leave it at this temperature, under stirring, for 6 hours. Filter using a buchner funnel and maintain the solid obtained, under low pressure, at a room temperature for 15 hours and then, at 40°C, for 5 hours.
Example 7 (comparative)
Precipitation
- [0063]
Disslove the sample in the suitable solvent (dichloromethane, acetone, ethyl acetate or ethanol) according to the ratios indicated in table 3 and then add the solvent, hexane or water, according to the ratios indicated in table 3, maintaining the mixture, under stirring, at room temperature. Recover the precipitate by filtration using a buchner funnel and desiccate as in example 6.
Example 8.
Obtaining a pharmaceutical form containing the medication in a defined crystalline form.
- [0064]
Prepare a fluid cream containing 2 % cetylic alcohol, 16% glyceryl monostearate, 10% vaseline oil, 13 % propylene glycol, 10% polyethylenglycol with low polymerization 1.5% polysorbate 80 and 47.5 % purified water. Add 1 g of cortexolone 17α-propionate of crystalline form III to 100 g of this cream and subject the mixture to homogenisation by means of a turbine agitator until you obtain homogeneity. You obtain a cream containing a fraction of an active ingredient dissolved in the formulation vehicle and a non-dissolved fraction of an active ingredient, present as a crystal of crystalline form III. This preparation is suitable for use as a formulation vehicle for skin penetration tests on Franz cells, where a coefficient of penetration in the range of 0.04 to 0.03 cm/h is observed on the preparation.
References
- ^ Jump up to:a b c d e f g h i j k l m n o p q r s t u v w “Winlevi (clascoterone) cream, for topical use”(PDF). Cassiopea. Retrieved 9 September 2020.
- ^ Jump up to:a b c http://adisinsight.springer.com/drugs/800026561
- ^ Jump up to:a b c Kircik LH (July 2019). “What’s new in the management of acne vulgaris”. Cutis. 104(1): 48–52. PMID 31487336.
- ^ Jump up to:a b Rosette C, Rosette N, Mazzetti A, Moro L, Gerloni M (February 2019). “Cortexolone 17α-Propionate (Clascoterone) is an Androgen Receptor Antagonist in Dermal Papilla Cells In Vitro”. J Drugs Dermatol. 18 (2): 197–201. PMID 30811143.
- ^ Jump up to:a b c Rosette C, Agan FJ, Mazzetti A, Moro L, Gerloni M (May 2019). “Cortexolone 17α-propionate (Clascoterone) Is a Novel Androgen Receptor Antagonist that Inhibits Production of Lipids and Inflammatory Cytokines from Sebocytes In Vitro”. J Drugs Dermatol. 18 (5): 412–418. PMID 31141847.
- ^ Jump up to:a b “Cassiopea Receives FDA Approval for Winlevi (clascoterone cream 1%), First-in-Class Topical Acne Treatment Targeting the Androgen Receptor”. Cassiopea (Press release). Retrieved 2020-08-30.
- ^ “Winlevi: FDA-Approved Drugs”. U.S. Food and Drug Administration (FDA). Retrieved 9 September 2020.
- ^ Jump up to:a b c d e f g h i j Barbieri, John S. (2020). “A New Class of Topical Acne Treatment Addressing the Hormonal Pathogenesis of Acne”. JAMA Dermatology. 156 (6): 619–620. doi:10.1001/jamadermatol.2020.0464. ISSN 2168-6068. PMID 32320045.
- ^ Jump up to:a b c d e Hebert A, Thiboutot D, Stein Gold L, Cartwright M, Gerloni M, Fragasso E, Mazzetti A (April 2020). “Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients With Facial Acne: Two Phase 3 Randomized Clinical Trials”. JAMA Dermatol. 156 (6): 621–630. doi:10.1001/jamadermatol.2020.0465. PMC 7177662. PMID 32320027.
- ^ Jump up to:a b c d e f Celasco G, Moro L, Bozzella R, Ferraboschi P, Bartorelli L, Quattrocchi C, Nicoletti F (2004). “Biological profile of cortexolone 17alpha-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist”. Arzneimittelforschung. 54 (12): 881–6. doi:10.1055/s-0031-1297043. PMID 15646372.
- ^ Jump up to:a b https://chem.nlm.nih.gov/chemidplus/rn/19608-29-8
- ^ Celasco G, Moroa L, Bozzella R, Ferraboschi P, Bartorelli L, Di Marco R, Quattrocchi C, Nicoletti F (2005). “Pharmacological profile of 9,11-dehydrocortexolone 17alpha-butyrate (CB-03-04), a new androgen antagonist with antigonadotropic activity”. Arzneimittelforschung. 55 (10): 581–7. doi:10.1055/s-0031-1296908. PMID 16294504.
- ^ Trifu V, Tiplica GS, Naumescu E, Zalupca L, Moro L, Celasco G (2011). “Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream”. Br. J. Dermatol. 165 (1): 177–83. doi:10.1111/j.1365-2133.2011.10332.x. PMID 21428978. S2CID 38404925.
- ^ Jump up to:a b c d e “Drug Trial Snapshot: Winlevi”. U.S. Food and Drug Administration (FDA). 26 August 2020. Retrieved 10 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ World Health Organization (2019). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82”. WHO Drug Information. 33 (3): 106. hdl:10665/330879.
- ^ Der Sarkissian SA, Sun HY, Sebaratnam DF (August 2020). “Cortexolone 17 α-proprionate for hidradenitis suppurativa”. Dermatol Ther: e14142. doi:10.1111/dth.14142. PMID 32761708.
External links
- “Clascoterone”. Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02608450 for “A Study to Evaluate the Safety and Efficacy of CB-03-01 Cream, 1% in Subjects With Facial Acne Vulgaris (25)” at ClinicalTrials.gov
- Clinical trial number NCT02608476 for “A Study to Evaluate the Safety and Efficacy of CB-03-01 Cream, 1% in Subjects With Facial Acne Vulgaris (26)” at ClinicalTrials.gov
 |
|
| Clinical data | |
|---|---|
| Trade names | Winlevi |
| Other names | CB-03-01; Breezula; 11-Deoxycortisol 17α-propionate; 17α-(Propionyloxy)- deoxycorticosterone; 21-Hydroxy-3,20-dioxopregn-4-en-17-yl propionate |
| License data |
|
| Routes of administration |
Topical (cream) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.810 |
| Chemical and physical data | |
| Formula | C24H34O5 |
| Molar mass | 402.531 g·mol−1 |
| 3D model (JSmol) | |
/////////Clascoterone, クラスコステロン , FDA 2020, 2020 APPROVALS, ANTI ACNE
[H][C@@]12CC[C@](OC(=O)CC)(C(=O)CO)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C