Clotrimazole
- Molecular FormulaC22H17ClN2
- Average mass344.837 Da
 |
|
Title: Clotrimazole
CAS Registry Number: 23593-75-1
CAS Name: 1-[(2-Chlorophenyl)diphenylmethyl]-1H-imidazole
Additional Names: 1-(o-chloro-a,a-diphenylbenzyl)imidazole; 1-[a-(2-chlorophenyl)benzhydryl]imidazole; 1-[(o-chlorophenyl)diphenylmethyl]imidazole; diphenyl-(2-chlorophenyl)-1-imidazolylmethane; 1-(o-chlorotrityl)imidazole
Manufacturers’ Codes: FB-5097; Bay b 5097
Trademarks: Canesten (Bayer); Canifug (Wolff); Empecid (Bayer-Takeda); Gyne-Lotrimin (Schering-Plough); Lotrimin (Schering-Plough); Mono-Baycuten; Mycelex-G (Miles); Mycofug (Hermal); Mycosporin (Bayer); Pedisafe (Sagitta); Rimazole (Cheil Sugar); Tibatin (Dak); Trimysten
Molecular Formula: C22H17ClN2
Molecular Weight: 344.84
Percent Composition: C 76.63%, H 4.97%, Cl 10.28%, N 8.12%
Literature References: Prepn: K. H. Buechel et al., ZA 6805392; eidem, US 3705172 (1969, 1972 both to Bayer). Pharmacology: Plempel et al., Antimicrob. Agents Chemother. 1969, 271; eidem, Dtsch. Med. Wochenschr. 94, 1356 (1969). Clinical findings: Oberste-Lehn et al., ibid. 1365. Series of articles on prepn, toxicology, pharmacokinetics, clinical studies: Arzneim.-Forsch. 22,1260-1272, 1276-1299 (1972). Toxicity: D. Tettenborn, ibid. 1276. Comprehensive description: J. G. Hoogerheide, B. E. Wyka, Anal. Profiles Drug Subs. 11, 225-255 (1982).
Properties: Crystals, mp 147-149°. A weak base, slightly sol in water, benzene, toluene; sol in acetone, chloroform, ethyl acetate, DMF. Hydrolyzes rapidly upon heating in aq acids. LD50 in male mice, rats (mg/kg): 923, 708 orally (Tettenborn).
Melting point: mp 147-149°
Toxicity data: LD50 in male mice, rats (mg/kg): 923, 708 orally (Tettenborn)
Derivative Type: Hydrochloride
Molecular Formula: C22H17ClN2.HCl
Molecular Weight: 381.30
Percent Composition: C 69.30%, H 4.76%, Cl 18.60%, N 7.35%
Properties: mp 159°.
Melting point: mp 159°
Therap-Cat: Antifungal.
Therap-Cat-Vet: Antifungal.
Keywords: Antifungal (Synthetic); Imidazoles.
|
Clotrimazole, sold under the brand name Canesten among others, is an antifungal medication.[1] It is used to treat vaginal yeast infections, oral thrush, diaper rash, pityriasis versicolor, and types of ringworm including athlete’s foot and jock itch.[1] It can be taken by mouth or applied as a cream to the skin or in the vagina.[1]
Common side effects when taken by mouth include nausea and itchiness.[1] When applied to the skin common side effects include redness and burning.[1] In pregnancy, use on the skin or in the vagina is believed to be safe.[1] There is no evidence of harm when used by mouth during pregnancy but this has been less well studied.[1] When used by mouth, greater care should be taken in those with liver problems.[1] It is in the azole class of medications and works by disrupting the cell membrane.[1]
Clotrimazole was discovered in 1969.[2] It is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system.[3] It is available as a generic medication.[1] The wholesale cost in the developing world as of 2014 is 0.20–0.86 USD per 20 gram tube of cream.[4] In the United States a course of treatment typically costs less than 25 USD.[5]
It is commonly available without a prescription in various dosage forms, such as a cream, vaginal tablet, or as a prescription troche or throat lozenge (prescription only). Topically, clotrimazole is used for vulvovaginal candidiasis (yeast infection) or yeast infections of the skin. For vulvovaginal candidiasis (yeast infection), clotrimazole tablets and creams are inserted into the vagina. Troche or throat lozenge preparations are used for oropharyngeal candidiasis (oral thrush) or prophylaxis against oral thrush in neutropenic patients.
Clotrimazole is usually used 5 times daily for 14 days for oral thrush, twice daily for 2 to 8 weeks for skin infections, and once daily for 3 or 7 days for vaginal infections.[6]
Clotrimazole may be compounded with a glucocorticoid, such as betamethasone, in a topical cream for the treatment of tinea corporis (ringworm), tinea cruris (jock itch) and tinea pedis (athlete’s foot). Although FDA approved, clotrimazole-betamethasone combination cream is not the preferred treatment for dermatophyte infections due to increased side effects from the topical glucocorticoid. Although temporary relief and partial suppression of symptoms may be observed with the combination therapy, glucocorticoids can elicit an immunosuppressive response and rebound effect that results in more severe infection typically requiring systemic antifungal agents to treat the disease. Combination creams are best avoided in order to improve treatment outcome, reduce the possibility of skin atrophy associated with prolonged topical glucocorticoid use, and to limit the cost of treatment. It can be effective in treating chronic paronychia. The preferred treatment of tinea infections is therefore with clotrimazole monotherapy.[7]
Topical and oral clotrimazole can be used in both adults and children.
Additionally, clotrimazole may be used to treat the sickling of cells (related to sickle cell anemia).[8][9]
Pregnancy
Small amounts of clotrimazole may be absorbed systemically following topical and vaginal administration. However, this may still be used to treat yeast infections in pregnant women.[10]
Side effects
Side effects of the oral formulation include itching, nausea, and vomiting. >10% of patients using the oral formulation may have abnormal liver function tests. Side effects include rash, hives, blisters, burning, itching, peeling, redness, swelling, pain or other signs of skin irritation.[1] For this reason, liver function tests should be monitored periodically when taking the oral clotrimazole (troche). When used to treat vulvovaginal candidiasis (yeast infection), <10% of patient have vulvar or vaginal burning sensation. <1% of patients have the following side effects: Burning or itching of penis of sexual partner; polyuria; vulvar itching, soreness, edema, or discharge [6][11][12]
Clotrimazole creams and suppositories contain oil which may weaken latex condoms and diaphragms.[10]
Drug interactions
There are no known significant drug interactions with topical clotrimazole. However, with oral (troche) clotrimazole, there are multiple interactions as the medication is a CYP450 enzyme inhibitor, primarily CYP3A4. Thus, any medication that is metabolized by the CYP3A4 enzyme will potentially have elevated levels when oral clotrimazole is used. The prescribing physician should be aware of any medication the patient is taking prior to starting oral clotrimazole. Certain medications should not be taken with oral clotrimazole.[11]
Mechanism of action
Clotrimazole works by inhibiting the growth of individual Candida or fungal cells by altering the permeability of the fungal cell wall. It binds to phospholipids in the cell membrane and inhibits the biosynthesis of ergosterol and other sterols required for cell membrane production.[12][11] Clotrimazole may be fungistatic (slow fungal growth) or fungicidal (result in fungal cell death).[1]
Society and culture
Clotrimazole (Canesten) antifungal cream
It is available as a generic medication.[1] The wholesale cost in the developing world as of 2014 is 0.20–0.86 USD per 20gm tube of cream.[4]In the United States a course of treatment typically costs less than 25 USD.[5] In 2016 Canesten was one of the biggest selling branded over-the-counter medications sold in Great Britain, with sales of £39.2 million.[13]
syn

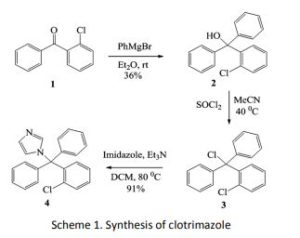
(2): Bromobenzene (1.6 g, 10.5 mmol) was added to
magnesium turnings (0.25 g, 10.5 mmol). The reaction
mixture was stirred at reflux and then at room
temperature for a further 6 hours. Ketone (1) (1.5 g,
7 mmol) was added and the reaction mixture was left
stirring at room temperature for overnight. The
reaction mixture was quenched with saturated
ammonium chloride solution (30 mL). The aqueous
layer was separated. Combined organic layers were
washed with water and dried over MgSO4 and then
removed. The crude yellow oily mass obtained was
purified by flash chromatography to give 0.75 g of
alcohol (2) (36% yield) as white crystals. mp 83-850C
(lit 83 0C)[7]; Rf = 0.45; IR (neat) νmax/cm-1 3566, 3058,
1466, 1010; 1H NMR (300 MHz, CDCl3) δ (ppm): 7.40
(dd, 1H, J = 1.6, 8.8 Hz), 7.24-7.35 (m, 11H), 7.01 (dt,
1H, J = 1.2, 6.3 Hz), 6.70 (dd, 1H, J = 1.5, 6.3 Hz), 4.46
(s, 1H); 13C NMR (75 MHz, CDCl3) δ (ppm): 145.6,
143.8, 133.3, 131.4, 131.3, 128.9, 127.9, 127.7, 127.2,
126.3, 82.5; m/z calcd for C19H14Cl [M-OH]+ 277.0784,
found 277.0782.
1H-imidazole (4): Thionyl chloride (0.82 g, 6.9 mmol)
was added into a solution of alcohol (2) (0.69 g, 2.3
mmol) and the mixture stirred at 0 0C for 1 hour. The
reaction mixture was left at reflux stirring for
overnight. The organic solvent was evaporated and
the residue washed with acetonitrile (2 x 20 mL) to
afford compound (3) as an yellow oily mass that was
submitted for the further reaction directly without
purification and structure elucidation. Imidazole (0.3
g, 4.4 mmol) with Et3N (0.6 g, 6.6 mmol) was added to
the solution of compound (3) (0.7 g, 2.2 mmol). The
reaction mixture was left at room temperature at
reflux stirring for 72 hours. The organic solvent was
removed and EtOAc (20 mL) with water (20 mL) was
added to the residue. The aqueous layer was
extracted with EtOAc (2 x 20 mL).
layers were washed with water and dried over MgSO4
prior to evaporation. The crude product was purified
by flash chromatography to afford 0.69 g of
compound (4) as a white crystal (yield 91%). mp 130-
133 0C; Rf = 0.37; IR (neat) νmax/cm-1 3064, 1489, 1443,
1210, 750; 1H NMR (300 MHz, CDCl3) δ (ppm): 7.48 (s,
1H), 7.41-7.44 (m 1H), 7.32-7.37 (m, 7H), 7.26-7.29
(m, 1H), 7.19-7.23 (m, 4H), 7.07 (s, 1H), 6.92 (dd, 1H, J
= 1.5, 6.3 Hz), 6.76 (s, 1H); 13C NMR (100 MHz, CDCl3)
δ (ppm): 151.1, 150.5, 148.9, 144.5, 140.3, 138.0,
137.7, 137.3, 135.5, 135.2, 135.1, 133.8, 127.0, 68.9;
m/z calcd for C19H14Cl [M-Imid]+ 277.0784, found
277.0780.
CLIP
Fig 4. Open Babel bond-line chemical structure with annotated hydrogens.
Click to toggle size.
Spectrum Plot
Fig 5. 1H NMR spectrum of C22H17ClN2 in CDCL3 at 400 MHz.

Figure 7. 2D 13 C13 C refocused INADEQUATE spectrum of clotrimazole showing intramolecular contacts among 13 C resonances as marked in the molecular structure on the right. The full spectrum is included in the Figure S4. The 2D spectrum was acquired in 17 hr at 106 K on 400 MHz, 384 scans per increment, 2 s recycle delay and 80 t 1 increments of a 27.7 ?s.


2D 13C-13C refocused INADEQUATE spectrum of clotrimazole showing intramolecular contacts among 13C resonances as marked in the molecular structure on the right. The 2D spectrum was acquired in 17 hr at 106 K on 400 MHz.

PATENT
https://patents.google.com/patent/CN105566156A/en
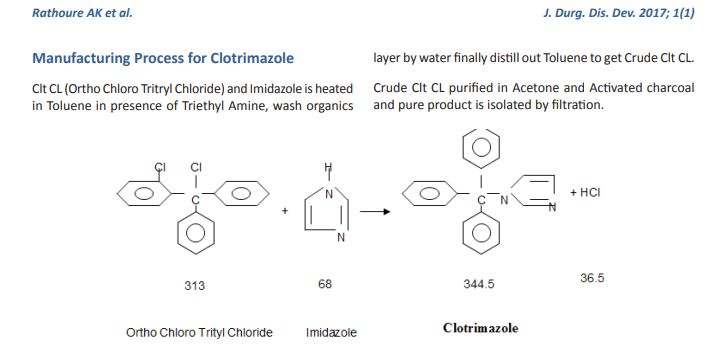
The object of the present invention is to provide a method for synthesizing a pharmaceutical Clotrimazole intermediate o-chlorobenzonitrile, comprising the steps of:
[0004] (i) in a reaction container equipped with a stirrer, a thermometer, a distillation apparatus, was added o-chlorobenzyl alcohol (2) 3. lmol, aniline (3) 3.6-3 · 9mol, nitromethane burning 310ml, chloro cuprous 1 · 56mol, hook are mixed, controlling the stirring speed 110-160rpm, the solution temperature increased to 110-115 ° C, 3-5h the reaction, the solution temperature increased to 130-135 ° C, the reaction 2-3h, solution temperature increased to 190-195 ° C, the reaction 90-120min, reducing the solution temperature to 15-20 ° C, was added 700 ml of saline solution, sodium bisulfite solution, 130ml, distilled under reduced pressure to collect 130-135 ° C fraction , washed with triethylamine in toluene and recrystallized to give crystals of o-chlorobenzonitrile (1).
[0005] wherein the mass fraction of nitromethane according to step (i) is 60-65%, of the salt solution in step (i) is ammonium nitrate, potassium iodide to any one of the steps of (i) mass fraction of sodium hydrogen sulfite solution was 40-45%, which pressure in the vacuum distillation of step (i) is 1.6-1.7kPa, triethylamine mass fraction of said step (i) is 70-75%, step (i) in toluene of the mass fraction of 90-95%. Throughout the reaction using the following reaction formula:
[0006
[0007 “not as good as Wu Ming 1 point Shi Bian: J Cheng less
A slave I anti Day “* 1, section A, J array low reaction temperature and reaction time, the reaction yield improved.
Detailed ways
[0008] The following examples with reference to specific embodiments of the present invention is further described:
Clotrimazole synthesis kinds drug intermediates of o-chlorobenzonitrile – [0009]
[0010] Example 1:
[0011] In a reaction vessel fitted with a stirrer, a thermometer, a distillation apparatus, was added o-chlorobenzyl alcohol (2) 3. Lmol, aniline (3) 3.6111〇1, mass fraction of 60% nitromethane 3,101,111 chloride cuprous 1.56111 〇1, mixing, stirring speed control lOrpm 1, the solution temperature increased to 110 ° C, the reaction 3h, the solution temperature increased to 130 ° C, the reaction 2h, the solution temperature is raised to 190 ° (:, reaction 9011 ^ 11, reducing the solution temperature to 15 ° (:, 7,001,111 ammonium nitrate solution was added, the mass fraction of 40% sodium bisulfite solution was 130ml, 1.6kPa vacuum distillation, collecting the fraction 130-135 ° C, mass fraction of 70 washed% triethylamine, 90% toluene to a mass fraction of recrystallized to give crystals of o-chlorobenzonitrile 308.02g, yield 72%.
[0012] Example 2:
[0013] In a reaction vessel fitted with a stirrer, a thermometer, a distillation apparatus, was added o-chlorobenzyl alcohol (2) 3. Lmol, aniline (3) 3.7111〇1, mass fraction of 62% nitromethane 31〇1111, 1.56111〇1 cuprous chloride, mixed, controlling the stirring speed of 130 rpm, the temperature was raised to 112 ° C, the reaction 4h, the solution temperature increased to 132 ° C, the reaction 2h, the solution temperature increased to 192 ° C, the reaction llOmin, reducing the solution temperature to 17 ° C, 700 ml of a solution of potassium iodide was added, the mass fraction of 42% sodium bisulfite solution 130ml, 1.65kPa vacuum distillation, collecting the fraction 130-135 ° C, mass fraction of 72% triethylamine washed, recrystallized from toluene to 92% mass fraction, to obtain crystals of o-chlorobenzonitrile 337.96g, yield 79%.
[0014] Example 3:
[0015] In a reaction vessel fitted with a stirrer, a thermometer, a distillation apparatus, was added o-chlorobenzyl alcohol (2) 3. Lmol, aniline (3) 3.9111〇1, mass fraction of 65% nitromethane 31〇1111, 1.56111 〇1 cuprous chloride, mixed, controlling stirring speed 160 rpm, temperature was raised to 115 ° C, the reaction 5h, the solution temperature increased to 135 ° C, the reaction 3h, the solution temperature increased to 195 ° C, the reaction 120min, reducing the solution temperature to 20 ° C, was added 700 ml of a solution of ammonium nitrate, 45% mass fraction of sodium bisulfite solution was 130ml, 1.7kPa vacuum distillation, collecting the fraction 130-135 ° C, mass fraction of 75% triacetyl amine scrubbing, 95%, recrystallized from toluene to a mass fraction to obtain crystals of o-chlorobenzonitrile 350.80g, yield 82%.
PATENT
https://patents.google.com/patent/US5091540A/en
Clotrimazole, i.e. 1-(o.Cl-α,α-diphenylbenzyl)imidazole, of formula: ##STR1## is a known antimycotic for human use, and a fungicide useful against plant pathogenic fungi.
Methods for its preparation are described in various patents. In particular, U.S. Pat. No. 3,929,820 describes a process starting from chlorophenyldiphenyl methylchloride and imidazole in the presence of a neutralizing agent, such as triethylamine, in a polar organic solvent. The process is strictly limited by the use, as the medium for the reaction in question, of a solvent falling within the given definition, i.e. having a dielectric constant of at least 4.5 and preferably between 15 and 50. In all the examples of the implementation of the process according to the patent in question, acetonitrile (D=37.5) is used as solvent.
EXAMPLE
900 g of benzene and 117.5 g of aluminium chloride are placed in a 2 liter flask fitted with a reflux condenser, stirrer and drying tube.
The mixture is cooled to 0° C. and a solution of 150 g of o.chlorobenzotrichloride in 150 g of benzene is added while maintaining a temperature not exceeding 15° C. The mixture is heated carefully under reflux for 4 hours. HCl is evolved.
The reaction mixture is then cooled to ambient temperature and slowly poured into 300 g of concentrated hydrochloric acid and 800 g of ice, so as not to exceed 25° C. The aqueous layer is then separated and discarded.
The benzene solution is washed with a solution of 230 g of sodium chloride in 800 g of water. The benzene phase is separated and dried over anhydrous sodium sulphate for 1 hour, and then filtered.
45 g of imidazole in 70 g of triethylamine are added to the filtrate and the mixture heated for 3 hours at 45°-50° C. It is then cooled to ambient temperature and 500 g of water are added while stirring. The aqueous layer is separated and discarded, and the benzene phase washed with 200 g of water. The benzene layer is separated and evaporated to dryness under vacuum.
The residue is dissolved in 250 g of ethyl acetate while stirring. 250 g of water are added and the solution titrated to calculate the exact quantity of nitric acid to add.
The solution is cooled to 15° C. and the calculated nitric acid quantity is quickly added. Stirring is halted when precipitation commences, and the system left until precipitation is complete.
The product is centrifuged and washed with 300 g of ethyl acetate and then with 300 g of water.
The moist product is placed into the reaction flask and 300 g of water, 450 g of methylene chloride, 5 g of triethylamine and 110 g of 30% sodium hydroxide are added. The mixture is stirred until a solution forms and the solution then left until the phases separate.
The aqueous phase is washed with 100 g of methylene chloride, and the pooled organic phases are washed twice with 200 g of water each time.
The solution in methylene chloride is treated with YMS decolorizing carbon and filtered, the filter then being washed with methylene chloride which si recovered by distillation. The residue is taken up in 100 g of acetone and redistilled to completely eliminate the methylene chloride.
The residue is taken up in 900 g of acetone and heated to 50° C. to obtain a complete solution. YMS decolorizing carbon and triethylamine are added, the mixture filtered and washed with acetone. Part of the acetone is then removed by distillation, reducing the volume to about 500 c.c. The mixture is cooled to 0° C. and, after five hours, the product is centrifuged and washed with 100 g of acetone. It is dried at 60° C., to obtain 150 g of final product.
References
- ^ Jump up to:a b c d e f g h i j k l m American Society of Health-System Pharmacists (8 February 2016). “Clotrimazole Monograph for Professionals”. www.drugs.com. Archived from the original on 28 October 2016. Retrieved 28 October 2016.
- ^ Walker, S. R. (2012). Trends and Changes in Drug Research and Development. Springer Science & Business Media. p. 109. ISBN 9789400926592. Archived from the original on 2016-09-14.
- ^ “WHO Model List of Essential Medicines (19th List)” (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ^ Jump up to:a b “Clotrimazole”. International Drug Price Indicator Guide. Archived from the original on 10 May 2017. Retrieved 28 October 2016.
- ^ Jump up to:a b Tarascon Pharmacopoeia 2016 Professional Desk Reference Edition. Jones & Bartlett Publishers. 2016. p. 176. ISBN 9781284095302. Archived from the original on 2016-10-28.
- ^ Jump up to:a b “Clotrimazole: MedlinePlus Drug Information”. The American Society of Health-System Pharmacists, Inc. Archived from the original on 18 April 2014. Retrieved 17 April2014.
- ^ Moriarty, B; Hay, R; Morris-Jones, R (10 July 2012). “The diagnosis and management of tinea”. BMJ (Clinical research ed.). 345: e4380. doi:10.1136/bmj.e4380. PMID 22782730.
- ^ Marieb & Hoehn, (2010). Human Anatomy and Physiology, p. 643. Toronto: Pearson
- ^ Rodgers, Griffin. “Hydroxyurea and other disease-modifying therapies in sickle cell disease”. UpToDate. Archived from the original on 15 April 2014. Retrieved 14 April2014.
- ^ Jump up to:a b “Diseases Characterized by Vaginal Discharge”. CDC. Archived from the original on 28 April 2014. Retrieved 17 April 2014.
- ^ Jump up to:a b c “Clotrimazole”. DrugBank. Archived from the original on 17 April 2014. Retrieved 17 April 2014.
- ^ Jump up to:a b “Clotrimazole (Oral)”. Lexicomp Online. Archived from the original on 23 January 2015. Retrieved 17 April 2014.
- ^ “A breakdown of the over-the-counter medicines market in Britain in 2016”. Pharmaceutical Journal. 28 April 2017. Retrieved 29 May 2017.
/////////////
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////