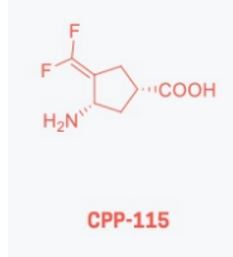
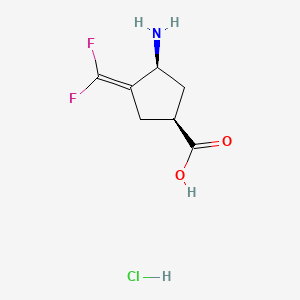
(+)-(1S,4S)-4-Amino-3-(difluoromethylene)-1-cyclopentanecarboxylic acid
640897-20-7 CAS
PHASE 1
NORTHWESTERN UNIVERSITY .INNOVATORS
| CPP-115 free base; UNII-5TD9324Z2U; CHEMBL146927; 640897-20-7; (1S,3S)-3-Amino-4-difluoromethylenyl-1-cyclopentanoic acid; (+)-(1S,4S)-4-Amino-3-(difluoromethylene)-1-cyclopentanecarboxylic acid | |
| Molecular Formula: | C7H9F2NO2 |
|---|---|
| Molecular Weight: | 177.151 g/mol |

- Originator Northwestern University
- Developer Catalyst Pharmaceutical Partners
- Class Aminobutyric acids; Antiepileptic drugs; Small molecules
- Mechanism of Action 4-aminobutyrate transaminase inhibitors
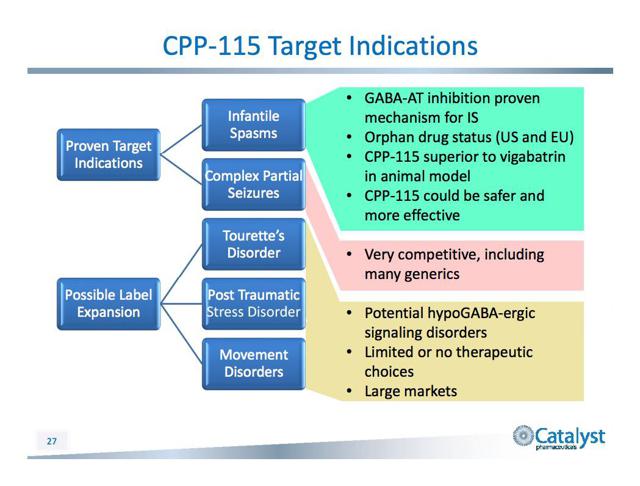
- Orphan Drug Status Yes – Infantile spasms
- On Fast track Drug abuse
- Cocaine Dependency
Highest Development Phases
- Phase I Gilles de la Tourette’s syndrome; Infantile spasms; Partial epilepsies
- Preclinical Drug abuse
Most Recent Events
- 19 Sep 2016 Efficacy data from a phase I trial in Infantile spasms released by Catalyst Pharmaceuticals
- 16 Dec 2015 Top-line adverse events and pharmacodynamics data from a phase Ib trial in Healthy volunteers released by Catalyst Pharmaceuticals
- 13 Oct 2015Catalyst Pharmaceuticals receives patent allowance for CPP 115 in USA

John Evans Professor of Chemistry, Northwestern University, Evanston, Illinois, USA.
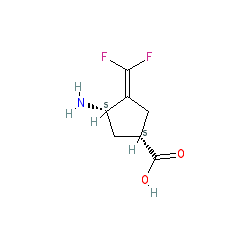
CPP 115 HCl salt, cas 760947-97-5
| UNII-0285I2MVUA; CPP-115; 760947-97-5; (1S,3S)-3-Amino-4-difluoromethylenyl-1-cyclopentanoic acid hydrochloride; Cyclopentanecarboxylic acid, 3-amino-4-(difluoromethylene)-, hydrochloride, (1S,3S)-; 0285I2MVUA | |
| Molecular Formula: | C7H10ClF2NO2 |
|---|---|
| Molecular Weight: | 213.609 g/mol |
Responsible Party:Catalyst Pharmaceuticals, Inc.ClinicalTrials.gov Identifier:NCT01493596 History of ChangesOther Study ID Numbers:CPP-115-0001 Study First Received:November 28, 2011Last Updated:May 10, 2012Health Authority:United States: Food and Drug Administration
Cpp-115: An Investigational Drug For Epilepsy
The fact that 1 in 12 people will have a seizure in their lifetime raises alarming signals to mitigate, prevent and cure epilepsy. The etiology is still unclear, but one of the pharmaceutical strategies to treat seizures is to replenish the local concentrations of GABA (gamma-aminobutyric acid, an inhibitory neurotransmitter in the human brain) that is degraded by an enzyme called GABA aminotransferase (GABA-AT). Mere consumption of GABA capsules is not effective, due to its inability to cross the blood-brain barrier (BBB). Therefore, an alternative strategy that involved stopping the function of GABA-AT was envisioned. Sabril is a first-in-class, FDA-approved antiepileptic drug; however, its daily dosage limit (1g – 3g) and adverse side effects, which include vision defects, call for further innovation.
Prof. Richard Silverman and his lab members at Northwestern University embarked on a scientific journey to identify BBB-penetrating antiepileptic compounds that would not cause visual defects. Through computational modeling and several cycles of optimization they discovered CPP-115 (chemical name: (1S,3S)-3-amino-4-difluoromethylene-1-cyclopentanoic acid; kinact/KI = 52 mM.min-1.)1 Mechanistically, CPP-115 binds to GABA-AT, undergoing product transformation that kills GABA-AT’s function. In rat studies, CPP-115 suppressed spasms at a much lower dose (0.1 mg/kg) than Sabril (>200 mg/kg) and exhibited better tolerance without visual defects.
CPP-115 (licensed to Catalyst Pharmaceuticals) elicited no cross-inhibition. It is metabolically more stable, with favorable PK characteristics (including rapid absorption and clearance). In a randomized, double-blind, single ascending dose phase I(a) study, CPP-115 was very well tolerated in all six doses (n=55 patients; maximum dose 500 mg, therapeutic dose 80 mg/day).2 Phase I(b) studies conducted in double-blind, placebo-controlled conditions demonstrated the safety and tolerability of CPP-115 in healthy volunteers. Intriguingly, an increase in brain GABA levels (150% to over 200%) was detected, accentuating CPP-115’s antiepileptic potential.2 Further clinical trials are currently in progress. CPP-115, with 12 years of unexpired patent life, has been granted orphan-drug designation in both the U.S. and EU for treating infantile spasms.
CPP-115 is one of a group of novel GABA-aminotransferase inhibitors discovered by scientists at Northwestern University. In 2009 Catalyst entered into a strategic collaboration with Northwestern University and in-licensed the worldwide rights to these inhibitors.
CPP-115 binds to GABA-AT (GABA-aminotransferase, also known as GABA transaminase or GABA-T), causing increased levels of GABA, gamma-aminobutyric acid, the chief inhibitory neurotransmitter in humans. It plays a role in regulating neuronal excitability throughout the nervous system. In humans, GABA is also directly responsible for the regulation of muscle tone.
In preclinical studies CPP-115 has been shown to have potentially significant advantages compared to the only approved and marketed current GABA-AT inhibitor (vigabatrin). CPP-115 may not cause the visual field defects associated with chronic administration of vigabatrin and it has been shown to be at least 200 times more potent in both in-vitro and animal model studies. The increased potency could enable the development of superior or alternative dosage forms and routes of administration. Catalyst hopes these important benefits will allow it to develop CPP-115 for a broad range of other central nervous system indications, such as infantile spasms, epilepsy, Tourette Syndrome and Post Traumatic Stress Disorder (PTSD). Additionally, Catalyst is exploring other selected diseases in which modulation of GABA levels might be beneficial. Catalyst believes that it controls all current intellectual property for GABA-aminotransferase inhibitors.
CPP-115 has received orphan drug designation in both the US and the EU for infantile spasms. Catalyst has begun the clinical development of CPP-115 by completing a randomized, double-blind, single ascending dose Phase I(a) study in normal healthy volunteers to evaluate the human safety characteristics of CPP-115, including CNS side effects and respiratory and cardiovascular safety. The Company reported results which indicated that CPP-115 was well tolerated at all six doses administered up to 500 mg, well above the anticipated therapeutic dose of up to 80 mg/day.
The hydrochloride salt of CPP-115 (PubChem CID 71252718) has been granted orphan drug designation by the EMA for the treatment of West syndrome, an epileptic disorder of young children which causes developmental problems. West syndrome is a long-term debilitating disease which may be life threatening as it can lead to severe damage to motor and cognitive functions. CPP-115 may have additional therapeutic applications for treating other neurological disorders, including drug addiction [4]. A single Phase I clinical trial has assessed CPP-115 as a treatment for cocaine addiction [3], but development has not progressed further.
Patent
| NORTHWESTERN UNIVERSITY [–/US]; 633 Clark Street Evanston, IL 60208 (US) | |
| Inventors: | SILVERMAN, Richard, B.; (US). ILAN, Yaron; (IL) |
Example 8
[0067] (IS, 4S)-6-Difluoromethylenyl-2-(4′-methoxybenzyl)-2- azabicyclo[2.2.1]heptan-3-one (13). At -78 °C, T uLi (1.7 M in pentane, 1.73 mL, 2.94 mmol) was slowly added to a stirred solution of diethyl (difluoromethyl)phosphonate (0.48 mL, 2.94 mmol) in anhydrous THF (15 mL). After being stirred for 0.5 h at -78 °C, 12 (0.60g, 2.45 mmol) in anhydrous THF (20 mL) was slowly added via syringe. Stirring continued for 1 h at – 78 °C , then the solution was allowed to warm to room temperature and heated to reflux for 24 h. Compound 12 is known and available in the art, and can be prepared as described in Qiu, J.; Silverman, R.B. A New Class of Conformationally Rigid Analogues of 4-Amino-5- halopentanoic Acids, Potent Inactivators of γ-Aminobutyric Acid Aminotransferase. J. Med. Chem. 2000, 43, 706-720. After the reaction had cooled down, THF was evaporated, and saturated NH4C1 solution (20 mL) was added to the residue, which was extracted with EtOAc (3 x 20 mL). The organic layer was washed with brine (2 x 20 mL), dried over anhydrous Na2S04, and concentrated under reduced pressure. The residue was purified by flash column
chromatography, eluting with hexanes/ethyl acetate (2: 1) to give 13 (0.47 g, 68%) as a colorless oil: 1H NMR (400 MHz, CDC13) δ 7.18 (d, J 8.4 Hz, 2H), 6.07 (d, J 8.4 Hz, 2H), 4.63 (d, J 14.8 Hz, 1H), 4.14 (s, 1H), 3.80 (s, 3H), 3.78 (d, J 14.8 Hz, 1H), 3.00 (s, 1H), 2.50 (dt, J 15.2, 3.6 Hz, 1H), 2.27 (dd, J 15.2, 2.4 Hz, 1H), 2.00 (d, J 9.2 Hz, 1H), 1.53 (d, 9.6 Hz, 1H); 13C NMR (100 MHz, CDC13) δ 177.37, 159.13, 152.19 (dd, J 285.7, 281.2 Hz), 129.59, 128.47, 1 14.13, 88.95 (dd, J 25.6, 22.2 Hz), 58.38 (d, J 5.3 Hz), 55.50, 45.60, 44.59, 40.96, 27.43; 19F NMR (376 MHz, CDC13) δ 42.64 and 41.01 (2 dd, J 60.2, 2.3 Hz, 2F). HRMS (EI) Ci5Hi5N02F2 calcd M
279.1071 , found M 279.10701.
Example 10
(IS, 3S)-3-Amino-4-difluoromethylenyl-l-cyclopentanoic acid (15) (i.e., compound 10, CPP-115, Figure 2). To lactam 14 (20.0 mg, 0.13 mmol) was added 4 mL of 4 N HCl. The solution was stirred at 70 °C for 10 h. After being washed with ethyl acetate (3 x 4 mL), the water layer was evaporated under reduced pressure to give a yellow solid. Recrystallization with ethanol/ether gave a white solid, which was then loaded on a cation- exchange column (AG50W-X8) and eluted with 0.2 N ammonium hydroxide to give the free amino acid 15 as a white solid (16 mg, 72%). 1H NMR (400 MHz, D20) δ 4.44 (s, 1H), 2.92 (m, 1H), 2.74 (m, 1H), 2.57 (dd, J 16.4, 3.6 Hz, 1H), 2.34 (m, 1H), 2.02 (d, J 14.8 Hz, 1H); 13C NMR (126 MHz, D20) δ 186.08, 155.30 (t, J 288.7 Hz), 92.19 (m), 53.16 (d, J 3.8 Hz), 48.01, 37.89, 32.45; 19F NMR (376 MHz, D20) δ -8.43 and -9.02 (2d, J 46.3 Hz, 2F); MS (ESI) C7H9N02F2 calcd M+H 178, found M+H 178.
PATENT
US 6794413
https://www.google.com/patents/US6794413
C7H11O2N, H% 7.85 C% 59.56 N% 9.92, found H% 7.88 C% 59.23 N% 9.62.
Example 5
(1S, 4S)-6-Difluoromethylenyl-2-(4′-methoxybenzyl)-2-azabicyclo [2.2.1]heptan-3-one (13). At −78° C., tBuLi (1.7 M in pentane, 1.73 mL, 2.94 mmol) was slowly added to a stirred solution of diethyl (difluoromethyl)phosphonate (0.48 mL, 2.94 mmol) in anhydrous THF (15 mL). After being stirred for 0.5 h at −78° C., 12 (0.60 g, 2.45 mmol) in anhydrous THF (20 mL) was slowly added via syringe. Stirring continued for 1 h at −78° C., then the solution was allowed to warm to room temperature and heated to reflux for 24 h. Compound 12 is known and available in the, art, and can be prepared as described in Qiu, J.; Silverman, R. B. A New Class of. Conformationally Rigid Analogues of 4-Amino-5-halopentanoic Acids, Potent Inactivators of γ-Aminobutyric Acid Aminotransferase. J. Med. Chem. 2000, 43, 706-720. After the reaction had cooled down, THF was evaporated, and saturated NH4Cl solution (20 mL) was added to the residue, which was extracted with EtOAc (3×20 mL). The organic layer was washed with brine (2×20 mL), dried4over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography, eluting with hexanes/ethyl acetate (2:1) to give 13 (0.47 g, 68%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 7.18 (d, J 8.4 Hz, 2H), 6.07 (d, J 8.4 Hz, 2H), 4.63 (d, J 14.8 Hz, 1H), 4.14 (s. 1H), 3.80 (s, 3H), 3.78 (d, J 14.8 Hz, 1H), 3.00 (s, 1H), 2.50 (dt, J 15.2, 3.6 Hz, 1H), 2.27 (dd, J 15.2, 2.4 Hz, 1H), 2.00 (d, J 9.2 Hz, 1H) 1.53 (d, 9.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 177.37, 159.13, 152.19 (dd, J 285.7, 281.2 Hz), 129.59, 128.47, 114.13, 88.95 (dd, J 25.6, 22.2 Hz), 58.38 (d, J 5.3 Hz), 55.50, 45.60, 44.59, 40.96, 27.43; 19F NMR (376 MHz, CDCl3) δ 42.64 and 41.01 (2 dd, J 60.2, 2.3 Hz, 2F). HRMS (EI) C15H15NO2F2 calcd M 279.1071, found M 279.10701.
Example 6
(1S, 4S)-6-Difluoromethylenyl-2-azabicyclo[2.2.1]heptan-3-one (14). Compound 13 (86.9 mg, 0.31 mmol) was dissolved in CH3CN (1.75 mL). A solution of ceric ammonium nitrate (512 mg, 0.93 mmol) in water (0.87 mL) was slowly added. The resulting solution was stirred at room temperature for 4 h. The reaction mixture was then diluted with ethyl acetate (20 mL), washed with brine (2×10 mL), and dried over anhydrous Na2SO4. After being concentrated under reduced pressure, the residue was purified by flash column chromatography, eluting with hexanes/ethyl acetate (1:1) to give the desired product as a colorless oil (33.6 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 5.48 (br s, 1H), 4.40 (s, 1H), 2.93 (s, 1H), 2.54 (dd, J 15.2, 2.8 Hz, 1H), 2.32 (d, J 15.2 Hz, 1H), 2.15 (d, J 9.6 Hz, 1H), 1.64 (d, J 10.0 Hz, 1H); 19F NMR (376 MHz, CDCl3) δ 42.85 and 40.00 (2d, J 60.2 Hz, 2F); HRMS (EI) C7H7NOF2 calcd M 159.0496, found M 159.04673.
Example 7
(1S, 3S)3-Amino-4-difluoromethylenyl-1-cyclopentanoic acid (15). To lactam 14 (20.0 mg, 0.13 mmol) was added 4 mL of 4 N HCl. The solution was stirred at 70° C. for 10 h. After being washed with ethyl acetate (3×4 mL), the water layer was evaporated under reduced pressure to give a yellow solid. Recrystallization with ethanol/ether gave a white solid, which was then loaded on a cation-exchange column (AG50W-X8) and eluted with 0.2 N ammonium hydroxide to give the free amino acid 15 as a white solid (16 mg, 72%). 1H NMR (400 MHz, D2O) δ 4.44 (s, 1H), 2.92 (m, 1H), 2.74 (m, 1H), 2.57 (dd, J 16.4, 3.6 Hz, 1H), 2.34 (m 1H), 2.02 (d, J 14.8 Hz, 1H); 13C NMR (126 MHz, D2O) δ 186.08, 155.30 (t, J 288.7 Hz), 92.19 (m), 53.16 (d, J 3.8 Hz), 48.01, 37.89, 32.45; 19F NMR (376 MHz, D2O) δ −8.43 and −9.02 (2d, J 46.3 Hz, 2F); MS (ESI) C7H9NO2F2 calcd M+H 178, found M+H 178.
paper
Journal of Medicinal Chemistry (2003), 46(25), 5292-5293
Design, Synthesis, and Biological Activity of a Difluoro-Substituted, Conformationally Rigid Vigabatrin Analogue as a Potent γ-Aminobutyric Acid Aminotransferase Inhibitor
Abstract

Previously it was found that a conformationally rigid analogue (2) of the epilepsy drug vigabatrin (1) did not inactivate γ-aminobutyric acid aminotransferase (GABA-AT). A cyclic compound with an exocyclic double bond (6) was synthesized and was found to inactivate GABA-AT, but only in the absence of 2-mercaptoethanol. The corresponding difluoro-substituted analogue (14) was synthesized and was shown to be a very potent time-dependent inhibitor, even in the presence of 2-mercaptoethanol.
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US2015196522 | METHODS OF USING (1S, 3S)-3-AMINO-4-DIFLUOROMETHYLENYL-1-CYCLOPENTANOIC ACID | 2015-03-02 | 2015-07-16 |
| US8969413 | Methods of using (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid | 2011-02-25 | 2015-03-03 |
| US2014336256 | METHOD OF TREATING TOURETTE’S DISORDER WITH GABA-AMINOTRANSFERASE INACTIVATORS | 2014-07-25 | 2014-11-13 |
| US2011237554 | Combination therapies: inhibitors of GABA transaminase and NKCC1 | 2011-09-29 | |
| US7381748 | Compounds and related methods for inhibition of gamma-aminobutyric acid aminotransferase | 2008-06-03 | |
| US6794413 | Compounds and related methods for inhibition of gamma-aminobutyric acid aminotransferase | 2004-09-21 |
RICHARD B. SILVERMAN
PROFESSOR
Research Statement
The research in my group can be summarized as investigations of the molecular mechanisms of action, rational design, and syntheses of potential medicinal agents, particularly for neurodegenerative diseases. Numerous drugs are known to function as specific inhibitors of particular enzymes. When little is known about the enzyme’s molecular mechanism of action, chemical model studies are designed to determine reasonable nonenzymatic pathways applicable to the enzyme. Based on the proposed mechanism of enzyme action, inhibitors are designed and synthesized. Organic synthesis is a primary tool for this work. The enzymes are isolated from either mammalian tissue or from overexpressed cells containing recombinant enzymes. Active site labeling studies utilize MALDI TOF and electrospray ionization mass spectrometry as well as radiolabeled inactivators and peptide mapping. We also are synthesizing compounds to act as receptor antagonists for important receptors related to neurodegenerative diseases.
Recent Publications
Lee, H.; Doud, E. H.; Wu, R.; Sanishvili, R.; Juncosa, J. I.; Liu, D.; Kelleher, N. L.; Silverman, R. B. Mechanism of inactivation of gamma-aminobutyric acid aminotransferase by (1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115). J. Am. Chem. Soc. 2015, 137, 2628-2640.
Zigmond, E.; Ya’acov, A. B.; Lee, H.; Lichtenstein, Y.; Shalev, Z.; Smith, Y.; Zolotarov, L.; Ziv, E.; Kalman, R.; Le, H. V.; Lu, H.; Silverman, R. B.; Ilan, Y. Suppression of hepatocellular carcinoma by inhibition of overexpressed ornithine aminotransferase. ACS Med. Chem. Lett. 2015, 6, 840-844.
Tang, W.; Li, H.; Doud, E. H.; Chen, Y.; Choing, S.; Plaza, C.; Kelleher, N. L.; Poulos, T. L.; Silverman, R. B. Mechanism of inactivation of neuronal nitric oxide synthase by (S)-2-amino-5-(2-(methylthio)acetimidamido)pentanoic acid. J. Am. Chem. Soc. 2015, 137, 5980-5989.
Le, H. V.; Hawker, D. D.; Wu, R.; Doud, E.; Widom, J.; Sanishvili, R.; Liu, D.; Kelleher, N. L.; Silverman, R. B. Design and mechanism of tetrahydrothiophene-based GABA aminotransferase inactivators. J. Am. Chem. Soc. 2015, 137, 4525-4533.
Huang, H.; Li, H.; Yang, S.; Chreifi, G.; Martásek, P.; Roman, L. J.; Meyskens, F. L.; Poulos, T. L.; Silverman, R. B. Potent and Selective Double-headed Thiophene-2-carboximidamide Inhibitors of Neuronal Nitric Oxide Synthase for the Treatment of Melanoma. J. Med. Chem. 2014, 57, 686-700.
Trippier, P. C.; Zhao, K. T.; Fox, S. G.; Schiefer, I. T.; Benmohamed, R.; Moran, J.; Kirsch, D. R.; Morimoto, R. I.; Silverman, R. B. Proteasome Activation is a Mechanism for Pyrazolone Small Molecules Displaying Therapeutic Potential in Amyotrophic Lateral Sclerosis. ACS Chem. Neurosci. 2014, 5, 823-829.
Holden, J. K.; Li, H.; Jing, Q.; Kang, S.; Richo, J.; Silverman, R. B.; Poulos, T. L. Structural and biological studies on bacterial nitric oxide synthase inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 18127-18131.
Kang, S.; Cooper, G.; Dunne, S. F.; Dusel, B.; Luan, C.-H.; Surmeier, D. J.; Silverman, R. B. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nature Commun 2012, 3, 1146.
Silverman, R. B. The 2011 E. B. Hershberg Award for Important Discoveries in Medicinally Active Substances: (1S,3S)-3-Amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA Aminotransferase Inactivator and New Treatment for Drug Addiction and Infantile Spasms. J. Med. Chem. 2012, 55, 567-575.
Chen, T.; Benmohamed, R.; Kim, J.; Smith, K.; Amante, D.; Morimoto, R. I.; Kirsch, D. R.; Ferrante, R. J.; Silverman, R. B. ADME-Guided Design and Synthesis of Aryloxanyl Pyrazolone Derivatives to Block Mutant SOD1 Cytotoxicity and Protein Aggregation: Potential Application for the Treatment of Amyotrophic Lateral Sclerosis. J. Med. Chem. 2012, 55, 515-527.
Selected Honors/Awards
- 2014 Fellow of the National Academy of Inventors
- 2014 Northwestern University Trustee Medal for Faculty Innovation and Entrepreneurship
- 2014 iCON Innovator Award (iBIO Institute)
- 2014 Elected to American Academy of Arts & Sciences
- 2014 Excellence in Medicinal Chemistry Prize of the Israel Chemical Society
- 2013 Fellow of the Royal Society of Chemistry (UK)
- 2013 Centenary Prize of the Royal Society of Chemistry
- 2013 Bristol-Myers Squibb-Edward E. Smissman Award of the American Chemical Society (ACS)
- 2013 Roland T. Lakey Award from Wayne State University
- 2012 Sato Memorial International Award of the Pharmaceutical Society of Japan
- 2011 Fellow of the ACS
- 2011 E. B. Hershberg Award for Important Discoveries in Medicinally Active Substances of the ACS
- 2011 Alumni Hall of Fame, Central High School of Central High School of Philadelphia
- 2009 Medicinal Chemistry Hall of Fame of the American Chemical Society
- 2009 Perkin Medal, Society of Chemical Industry
- 2008 Alumni Fellow Award, Pennsylvania State University
- 2003 Arthur C. Cope Senior Scholar Award of the American Chemical Society
- 2000 Northwestern University Alumni Association Excellence in Teaching Award
- 1999 E. LeRoy Hall Award for Teaching Excellence
- 1999 Excellence in Chemistry Education Award from the Northwestern University Chapter of Alpha Chi Sigma Chemistry Fraternity
- 1990 Fellow of the American Association for the Advancement of Science
- 1985 Fellow of the American Institute of Chemists
- 1982 NIH Research Career Development Awardee
- 1981 Alfred P. Sloan Research Fellow
- 1976 Du Pont Young Faculty Fellow
 Silverman describes the structure of pregabalin.
Silverman describes the structure of pregabalin.
In recognition of his outstanding work in applied chemistry, the Society of Chemical Industry 2009 Perkin Medal has been awarded to Richard B. (Rick) Silverman, the John Evans Professor of Chemistry at Northwestern University. The Perkin Medal, which was first awarded just over one century ago, is recognized as one of the chemical industry’s most prestigious awards.
Silverman’s research primarily focuses on medicinal chemistry: studying the molecular basis of drug action, reaction mechanisms of enzymes, and design and synthesis of pharmaceutical agents. He has worked to deepen understanding of several diseases, including epilepsy, cancer, Parkinson’s, and cerebral palsy.
Among Silverman’s many scientific accomplishments, designing pregabalin and discovering the medicinal properties of that compound stand out for catapulting him and Northwestern to pharmaceutical fame and fortune. Pregabalin, a γ-aminobutyric acid analog, is the active substance in Lyrica, a pain and epilepsy medication commercialized by drug giant Pfizer.
In 2007, after Northwestern collected more than $70 million in royalties for the drug, the university sold a portion of its royalty rights for an additional $700 million (C&EN, March 10, 2008, page 56). Around the same time, Silverman and his family donated a portion of their earnings from the drug to fund construction of a new Northwestern science building. The facility, which is scheduled to open this fall, will house chemistry, biology, and engineering research groups devoted to biomedical science.
Silverman has published more than 250 papers in organic chemistry, medicinal chemistry, and enzymology. He is also the author of three books, including “The Organic Chemistry of Drug Design and Drug Action,” and holds 40 patents.
The Perkin Medal is named for Sir William Henry Perkin (1838–1907), who was honored by SCI in 1906 for developing the first synthetic dye, Perkin mauve. This year’s medal will be presented at SCI’s Perkin Medal banquet in Philadelphia in September.
The Legacy Of Lyrica
Northwestern’s Richard Silverman, professor of chemistry, developed pregabalin, the chemical that Pfizer now markets as Lyrica. The drug is one of the two approved treatments for fibromyalgia, epilepsy, and the most effective treatment for seizures as well.
In his laboratory, Silverman’s research team studied chemicals made in the brain. Of particular interest was GABA, a neurotransmitter that inhibits certain brain functions. When GABA levels fall too low in some people, it can trigger epileptic seizures. His group studied enzymes that affect GABA levels, looking for ways to keep GABA elevated. In 1989, the Parke-Davis unit of Warner-Lambert was interested in the research findings. Among the 17 chemical analogs that Silverman sent to Parke-Davis, only pregabalin showed effects in mice.
Serendipity played a huge part in shaping this success story, as most chemicals that affect cells in lab experiments do not survive inside an animal. Another outcome of the research was that the compound was effective for a reason entirely different from Silverman’s initial goal of producing more GABA. In another stroke of luck, the molecule happened to be of the right shape to be transported directly into the brain with nearly 90 percent efficacy.
Lyrica has been a tremendous medical and commercial success that has validated the nearly 15 year process from invention to market launch in 2005. In 2004 Lyrica was approved for use in adults for the treatment of various peripheral neuropathic pain indications as well as therapy for partial epilepsy in more than 60 countries outside of the United States. In 2006 Lyrica was also approved for the treatment of generalized anxiety disorder in Europe. The drug brought in $1.2 billion in sales in 2006 and in 2010 was approved in Europe to treat central neuropathic (nerve) pain. This is expected to push profits from the blockbuster drug to climb even higher.
Northwestern sold a sizeable amount of royalty interest in 2007 to Royalty Pharma, a company that specializes in acquiring cash-generating intellectual property, for $700 million to help the university’s endowment. This deal has been termed the largest sale ever of a royalty stream for a pharmaceutical product.
To learn more about Lyrica visit the product website at www.lyrica.com.
////////Cocaine Dependency, CPP 115, PHASE 1, CATALYST, NORTHWESTERN UNIVERSITY, ORPHAN DRUG, 640897-20-7, 760947-97-5
C1C(CC(=C(F)F)C1N)C(=O)O