Cyclobenzaprine
- Molecular FormulaC20H21N
- Average mass275.387 Da
- MK-130
- TNX-102
Cyclobenzaprine (sold under the brand name Flexeril, among others) is a medication used for muscle spasms from musculoskeletal conditions of sudden onset.[6] It is not useful in cerebral palsy.[6] It is taken by mouth.[6] Use is not recommended for more than a few weeks.[6]
Common side effects include headache, feeling tired, dizziness, and dry mouth.[6] Serious side effects may include an irregular heartbeat.[6] There is no evidence of harm in pregnancy, but it has not been well studied in this population.[6] It should not be used with an MAO inhibitor.[6] How it works is unclear.[6]
Cyclobenzaprine was approved for medical use in the United States in 1977.[6] It is available as a generic medication.[6] In 2019, it was the 45th most commonly prescribed medication in the United States, with more than 15 million prescriptions.[7][8] It was not available in the United Kingdom as of 2012.[9]
Synthesis Reference
Villani, F.J.; US. Patent 3,409,640; November 5,1968; assigned to Schering Corporation.
Paper
A simple and convenient protocol for deoxygenation of aliphatic and aromatic N-oxides to the corresponding amines in good to excellent yield using sodium borohydride–Raney nickel in water is reported. Other functional moieties such as alkenes, halides, ethers, and amides are unaffected under the present reaction condition.
Graphical abstract

Cyclobenzaprine N-oxide, CAS RN: 6682-26-4
Dissolve (1 mmol) of cyclobenzaprine N-oxide in 2.5 mL of water at 60 °C. 2. Add Raney nickel (0.10 g, W6 grade) to the solution. 3. Stir the reaction mixture for 10 minutes. 4. Add (2 mmol) of sodium borohydride slowly in portions over 15-20 minutes to the reaction mixture. 5. Stir the reaction mixture at the same temperature for 2.5 hours (the completion of the reaction as monitored by TLC). 6. Once the reaction is completed, add chloroform (50 mL) to the reaction mixture. 7. Filter the resulted mixture to remove Raney nickel. 8. Dry the chloroform layer over anhydrous magnesium sulfate. 9. Filter the reaction mixture. 10. Evaporate the solvent under vacuum. 11. Purify the obtained residue through short path flash chromatography with silica gel and chloroform.
1H NMR (400 MHz, CDCl3) δ: 1.12 (s, 6H, N-CH3), 1.23- 1.34 (m, 4H, CH2), 4.58 (t, J= 4.0 Hz, 1H, CH), 5.82(d, J= 4.0 Hz, 2H, CH), 6.21- 6.33 (m, 8H, ArH).
13C NMR (100 MHz, CDCl3) δ: 27.89, 45.93, 60.12, 127.40, 127.55, 128.30, 128.59, 128.92, 129.33, 129.45, 129.67, 131.74, 131.96, 132.40, 134.63, 135.39, 137.97, 142.95, 143.30.
SYN
PATENT
https://patents.google.com/patent/WO2012098563A2/en

PATENT
PATENT
CN 111393305
CLIP
Muscle Relaxants
R.S. Vardanyan, V.J. Hruby, in Synthesis of Essential Drugs, 2006
Cyclobenzaprine
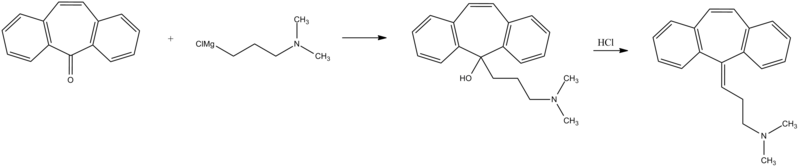
Cyclobenzaprine, N,N-dimethyl-3-(dibenzo[a,d]cyclohepten-5-ylidene) propylamine (15.3.9), is synthesized by reacting 5H-dibenzo[a,d]cyclohepten-5-one with 3-dimethylaminopropylmagnesium chloride and subsequent dehydration of the resulting carbinol (15.3.8) in acidic conditions into cyclobenzaprine (15.3.9) [30–32].


Cyclobenzaprine is structurally similar to tricyclic antidepressants. It acts at the brain stem level. It is used as an adjuvant agent for relieving muscle spasms associated with severe diseased conditions of the muscle. A synonym of this drug is flexeril.
///////////////////////////////////////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Medical use
Cyclobenzaprine is used, in conjunction with physical therapy, to treat muscle spasms that occur because of acute musculoskeletal conditions.[10] After sustaining an injury, muscle spasms to stabilize the affected body part occur, which may increase pain to prevent further damage. Cyclobenzaprine is used to treat such muscle spasms associated with acute, painful musculoskeletal conditions.[11] It decreases pain in the first two weeks,[12][13] peaking in the first few days, but has no proven benefit after two weeks.[12][14] Since no benefit is proven beyond that, therapy should not be continued long-term.[11] It is the best-studied muscle relaxer.[12] It is not useful for spasticity due to neurologic conditions such as cerebral palsy.[11][15]
A 2004 review found benefit for fibromyalgia symptoms, with a reported number needed to treat of 4.8 (meaning that 1 person out of every 4.8 benefits from treatment) for pain reduction, but no change in fatigue or tender points.[16] A 2009 Cochrane review found insufficient evidence to justify its use in myofascial pain syndrome.[17] It may also be used along with other treatments for tetanus.[18]
Side effects
Cyclobenzaprine results in increased rates of drowsiness (38%), dry mouth (24%), and dizziness (10%).[14] Drowsiness and dry mouth appear to intensify with increasing dose.[19] The sedative effects of cyclobenzaprine are likely due to its antagonistic effect on histamine, serotonin, and muscarinic receptors.[medical citation needed]
Agitation is a common side effect observed, especially in the elderly. Some experts[who?] believe that cyclobenzaprine should be avoided in elderly patients because it can cause confusion, delirium, and cognitive impairment.[20][21] In general, the National Committee for Quality Assurance recommends avoiding the use of cyclobenzaprine in the elderly because of the potential for more severe side effects.[22]
Dysphagia, a life-threatening side-effect, may rarely occur.[23] Treatment protocols and support should follow the same as for any structurally related tricyclic, such as tricyclic antidepressants.[24]
Overdose
The most common effects of overdose are drowsiness and tachycardia.[11] Rare but potentially critical complications are cardiac arrest, abnormal heart rhythms, severe low blood pressure, seizures, and neuroleptic malignant syndrome.[11] Life-threatening overdose is rare,[11] however, as the median lethal dose is about 338 milligrams/kilogram in mice and 425 mg/kg in rats.[11] The potential harm is increased when central nervous system depressants and antidepressants are also used; deliberate overdose often includes alcohol among other drugs.[11]
Interactions
Cyclobenzaprine has major contraindications with monoamine oxidase inhibitors (MAOIs). At least one study also found increased risk of serotonin syndrome when cyclobenzaprine was taken with the serotonergic drugs duloxetine or phenelzine.[25]
These substances may interact with cyclobenzaprine:
- Central nervous system depressants (e.g. alcohol, opioids, benzodiazepines, nonbenzodiazepines, phenothiazines, carbamates, barbiturates, major tranquilizers)
- Monoamine oxidase inhibitors taken within two weeks of cyclobenzaprine may result in serious, life-threatening side effects.[11]
Cyclobenzaprine may affect the medications used in surgical sedation and some surgeons request that patients temporarily discontinue its use prior to surgery.[26]
Pharmacology
Cyclobenzaprine is a centrally acting muscle relaxant.[27] Cyclobenzaprine is a 5-HT2 receptor antagonist; it relieves muscle spasm through action on the central nervous system at the brain stem, rather than targeting the peripheral nervous system or muscles themselves.[28]
Pharmacodynamics
| Site | CBP | NCBP | Action | Ref |
|---|---|---|---|---|
| 5-HT1A | 5.3 | 3.2 | Agonist | [29] |
| 5-HT2A | 5.2 | 13 | Antagonist | [29] |
| 5-HT2B | 100 | ??? | Antagonist | [29] |
| 5-HT2C | 5.2 | 43 | Antagonist | [29] |
| α1A | 5.6 | 34 | ND | [29] |
| α2A | 4.3 | 6.4 | Antagonist | [29] |
| α2B | 21 | 150 | ND | [29] |
| α2C | 21 | 48 | ND | [29] |
| H1 | 1.3 | 5.6 | ND | [29] |
| M1 | 7.9 | 30 | ND | [29] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | ||||
Pharmacokinetics
Cyclobenzaprine has an oral bioavailability of about 55% and approximately 93% is bound to proteins in plasma. The half-life of the drug is 18 hours and it has a plasma clearance of 0.7 litres per minute.[27][30][31]
Comparison to other medications
Cyclobenzaprine has been found to be not inferior to tizanidine, orphenadrine, and carisoprodol in the treatment of acute lower back pain, although none have been proven to be effective for long-term use (beyond two weeks of treatment). No differences in pain or spasm scores were noted among these agents, nor when compared to benzodiazepines.[32] However, nonbenzodiazepine (including cyclobenzaprine) treatment was found to have a lower risk of medication abuse and continuation of use against medical advice.[medical citation needed] Side effects such as sedation and ataxia are also less pronounced with nonbenzodiazepine antispasmodics.[medical citation needed]
In a study on the treatment of musculoskeletal pain treatment with cyclobenzaprine alone or in combination with ibuprofen, no significant differences in pain scores were noted among the three treatment groups. Peak benefit was found to occur on day seven of the treatment for all groups.[33]
Formulations
By mouth, cyclobenzaprine is marketed as Apo-Cyclobenzaprin, Fexmid, Flexeril and Novo-Cycloprine. It is available in generic form. A once-a-day, extended-release formulation, Amrix, is available.[34] Cyclobenzaprine is also used by compounding pharmacies in topical creams.[citation needed]
References
- ^ Micromedex® 2010 – DRUGDEX Evaluations (Cyclobenzaprine Hydrochloride)
- ^ “Cyclobenzaprine Hydrochloride Tablets USP Revised: April 2005 Rx only”. nih.gov. Retrieved 1 October 2016.
- ^ Teva Pharmaceuticals USA, Inc (May 2016). “AMR40470 (Amrix) Prescribing Information” (PDF).
- ^ U.S. Food and Drug Administration. “NDA 17-821/S-045 Flexeril (Cyclobenzaprine HCl) Tablets” (PDF).
- ^ Teva Pharmaceuticals USA, Inc (May 2016). “AMR40470 (Amrix) Prescribing Information” (PDF).
- ^ Jump up to:a b c d e f g h i j k “Cyclobenzaprine Monograph for Professionals”. Drugs.com. AHFS. Retrieved 22 December 2018.
- ^ “The Top 300 of 2019”. ClinCalc. Retrieved 16 October 2021.
- ^ “Cyclobenzaprine – Drug Usage Statistics”. ClinCalc. Retrieved 16 October 2021.
- ^ “Fibromyalgia, psychiatric comorbidity, and the somatosensory cortex”. British Journal of Medical Practitioners. 5 (2): a522. 2012.
- ^ Yang YW, Macdonald JB, Nelson SA, Sekulic A (December 2017). “Treatment of vismodegib-associated muscle cramps with cyclobenzaprine: A retrospective review”. Journal of the American Academy of Dermatology. 77 (6): 1170–1172. doi:10.1016/j.jaad.2016.12.017. PMID 29132849. S2CID 8265576.
- ^ Jump up to:a b c d e f g h i “Cyclobenzaprine- cyclobenzaprine hydrochloride tablet, film coated”. DailyMed. 30 December 2019. Retrieved 26 September 2020.
- ^ Jump up to:a b c Chou R, Peterson K, Helfand M (August 2004). “Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review”. Journal of Pain and Symptom Management. 28 (2): 140–75. doi:10.1016/j.jpainsymman.2004.05.002. PMID 15276195.
- ^ van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM (2003). Van Tulder MW (ed.). “Muscle relaxants for non-specific low back pain”. The Cochrane Database of Systematic Reviews. 2 (2): CD004252. doi:10.1002/14651858.CD004252. PMC 6464310. PMID 12804507.
- ^ Jump up to:a b Browning R, Jackson JL, O’Malley PG (July 2001). “Cyclobenzaprine and back pain: a meta-analysis”. Archives of Internal Medicine. 161 (13): 1613–20. doi:10.1001/archinte.161.13.1613. PMID 11434793.
- ^ Ashby P, Burke D, Rao S, Jones RF (October 1972). “Assessment of cyclobenzaprine in the treatment of spasticity”. Journal of Neurology, Neurosurgery, and Psychiatry. 35 (5): 599–605. doi:10.1136/jnnp.35.5.599. PMC 494138. PMID 4563483.
- ^ Tofferi JK, Jackson JL, O’Malley PG (February 2004). “Treatment of fibromyalgia with cyclobenzaprine: A meta-analysis”. Arthritis and Rheumatism. 51 (1): 9–13. doi:10.1002/art.20076. PMID 14872449.
- ^ Leite FM, Atallah AN, El Dib R, Grossmann E, Januzzi E, Andriolo RB, da Silva EM (July 2009). “Cyclobenzaprine for the treatment of myofascial pain in adults”. The Cochrane Database of Systematic Reviews (3): CD006830. doi:10.1002/14651858.CD006830.pub3. PMC 6481902. PMID 19588406.
- ^ Smith BT (2014). Pharmacology for Nurses. Jones & Bartlett Publishers. p. 122. ISBN 9781449689407.
- ^ “Flexeril: Side effects”. RxList.com. Archived from the original on 12 September 2008. Retrieved 22 February 2010.
- ^ “Long-term Use of Cyclobenzaprine for Pain: A Review of the Clinical Effectiveness”. CADTH Rapid Response Reports. Ottawa, Ontario: Canadian Agency for Drugs and Technologies in Health. 23 February 2015. PMID 25763449.
- ^ Potentially inappropriate medications for the elderly according to the revised Beers criteria. 2012. Duke Clinical Research Institute website. [1]
- ^ “High risk medications” (PDF). National Committee for Quality Assurance. Archived from the original (PDF) on 1 February 2010. Retrieved 22 February 2010.
- ^ “MEDICATIONS AND DYSPHAGIA/ SWALLOWING RISKS” (PDF).
- ^ Chabria SB (July 2006). “Rhabdomyolysis: a manifestation of cyclobenzaprine toxicity”. Journal of Occupational Medicine and Toxicology. 1 (1): 16. doi:10.1186/1745-6673-1-16. PMC 1540431. PMID 16846511.
- ^ Keegan MT, Brown DR, Rabinstein AA (December 2006). “Serotonin syndrome from the interaction of cyclobenzaprine with other serotoninergic drugs”. Anesthesia and Analgesia. 103 (6): 1466–8. doi:10.1213/01.ane.0000247699.81580.eb. PMID 17122225.
- ^ Medical Practice of William H. Gorman, M.D. (18 February 2014). “Medications to Avoid, Continue, or Stop – Before & After Surgery”.
- ^ Jump up to:a b “Cyclobenzaprine”. www.drugbank.ca.
- ^ Kobayashi H, Hasegawa Y, Ono H (September 1996). “Cyclobenzaprine, a centrally acting muscle relaxant, acts on descending serotonergic systems”. European Journal of Pharmacology. 311 (1): 29–35. doi:10.1016/0014-2999(96)00402-5. PMID 8884233.
- ^ Jump up to:a b c d e f g h i j k “Cyclobenzaprine (CBP) and Its Major Metabolite Norcyclobenzaprine (nCBP) Are Potent Antagonists of Human Serotonin Receptor 2a (5HT2a), Histamine Receptor H-1 and á-Adrenergic Receptors: Mechanistic and Safety Implications for Treating Fibromyalgia Syndrome by Improving Sleep Quality”. ACR Meeting Abstracts. Retrieved 27 January 2022.
- ^ “Cyclobenzaprine”. pubchem.ncbi.nlm.nih.gov.
- ^ Winchell GA, King JD, Chavez-Eng CM, Constanzer ML, Korn SH (January 2002). “Cyclobenzaprine pharmacokinetics, including the effects of age, gender, and hepatic insufficiency”. Journal of Clinical Pharmacology. 42 (1): 61–9. doi:10.1177/0091270002042001007. PMID 11808825. S2CID 7749001.
- ^ “Medscape: Medscape Access”. medscape.com. Retrieved 1 October 2016.
- ^ Childers MK, Petri M, Laudadio C, Harrison D, Silber S, Bowen D (2004). “Comparison of cyclobenzaprine alone versus cyclobenzaprine plus ibuprofen in patients with acute musculoskeletal spasm and pain”. Annals of Emergency Medicine. 44 (4): S87–S88. doi:10.1016/j.annemergmed.2004.07.286.
- ^ “Patient Web site for Amrix (Cyclobenzaprine Hydrochloride Extended‐Release Capsules)”. amrix.com. Retrieved 1 October 2016.
External links
- “Cyclobenzaprine”. Drug Information Portal. U.S. National Library of Medicine.
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Flexeril, Amrix, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682514 |
| License data | |
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 33–55%[1][2] |
| Protein binding | 93% |
| Metabolism | major: CYP3A4, CYP1A2; minor: CYP2D6, N-demethylation[5] |
| Metabolites | Norcyclobenzaprine |
| Elimination half-life | 32 hours (extended-release, range 8-37 hours),[3] 18 hours (immediate release, range 8–37 hours)[4] |
| Excretion | Kidney |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.588 |
| Chemical and physical data | |
| Formula | C20H21N |
| Molar mass | 275.395 g·mol−1 |
| 3D model (JSmol) | |
| (verify) | |
///////////////cyclobenzaprine, циклобензаприн , سيكلوبنزابرين , 环苯扎林 , MK-130, TNX-102, Muscle Relaxant
CN(C)CCC=C1C2=CC=CC=C2C=CC2=CC=CC=C12