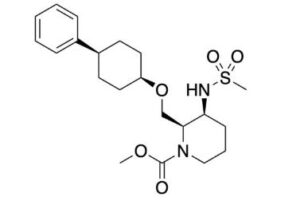
Danavorexton, TAK 925
2114324-48-8
- Molecular FormulaC21H32N2O5S
- Average mass424.554 Da
-
- OriginatorTakeda
- ClassCyclohexanes; Esters; Ethers; Piperidines; Sleep disorder therapies; Small molecules; Sulfonamides
- Mechanism of ActionOrexin receptor type 2 agonists
- Orphan Drug StatusYes – Narcolepsy
- Phase IHypersomnia; Narcolepsy; Respiration disorders; Sleep apnoea syndrome
- 01 Jun 2022Takeda Pharmaceuticals completes a phase I clinical trials in Respiratory disorder (In adults) in Netherlands (IV) (ISRCTN63027076)
- 02 Apr 2022Efficacy and safety data from phase a Ib trial in Hypersomnia presented at the 74th Annual Meeting of the American Academy of Neurology 2022 (AAN-2022)
- 10 Mar 2022Phase-I clinical trials in Sleep apnoea syndrome in Australia (IV) (NCT05180890)
Danavorexton (developmental code name TAK-925) is a selective orexin 2 receptor agonist.[1] It is a small-molecule compound and is administered intravenously.[1][2] The compound was found to dose-dependently produce wakefulness to a similar degree as modafinil in a phase 1 clinical trial.[1][3] As of March 2021, danavorexton is under development for the treatment of narcolepsy, idiopathic hypersomnia, and sleep apnea.[2][1][4] It is related to another orexin receptor agonist known as TAK-994, the development of which was discontinued for safety reasons in October 2021.[1][5]
PAPER
https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00626
TAK-925, a potent, selective, and brain-penetrant orexin 2 receptor (OX2R) agonist, [methyl (2R,3S)-3-((methylsulfonyl)amino)-2-(((cis-4-phenylcyclohexyl)oxy)methyl)piperidine-1-carboxylate, 16], was identified through the optimization of compound 2, which was discovered by a high throughput screening (HTS) campaign. Subcutaneous administration of compound 16 produced wake-promoting effects in mice during the sleep phase. Compound 16 (TAK-925) is being developed for the treatment of narcolepsy and other related disorders.

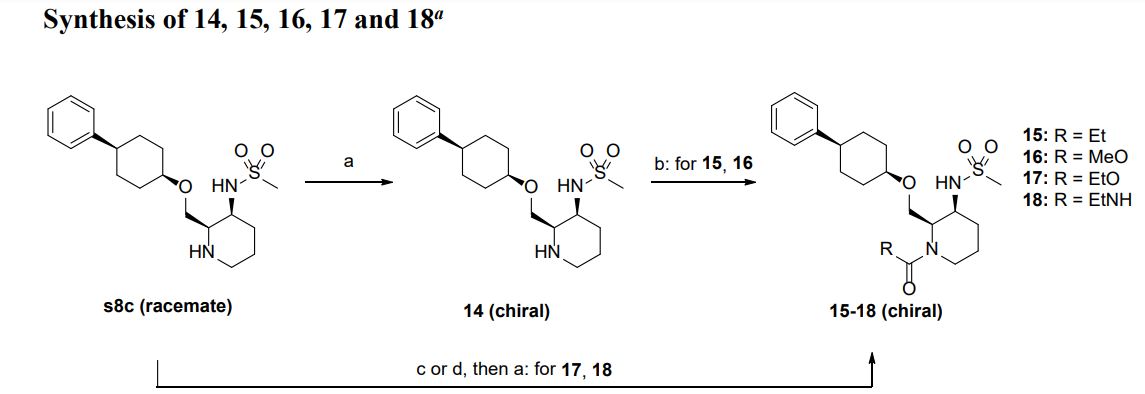
aReagents and conditions: (a) chiral column separation; (b) RCOCl, Et3N, THF, rt (for 15 and 16); (c) ethyl chlorocarbonate, DIEA, THF, rt (for 17); (d) isocyanatoethane, Et3N, THF, 0 °C−rt (for 18).
Methyl (2R,3S)-3-((methylsulfonyl)amino)-2-(((cis-4- phenylcyclohexyl)oxy)methyl)piperidine-1-carboxylate (16) To a mixture of 14 (58 mg, 0.16 mmol) and Et3N (0.044 mL, 0.32 mmol) in THF (3 mL) was added methyl chlorocarbonate (0.024 mL, 0.32 mmol) at rt. The mixture was stirred at rt overnight. The mixture was quenched with water and extracted with EtOAc. The organic layer was separated, washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexane/EtOAc, 1:1 to 0:100) to give 16 (64 mg, 0.15 mmol, 95%) as a colorless oil. Crystallization of 16 (1.8 g, 4.1 mmol) from EtOH-H2O gave 16 (1.7 g, 3.9 mmol, 95%) as a white solid. 1H NMR (600 MHz, DMSO-d6) δ 1.40−1.55 (5H, m), 1.56−1.73 (5H, m), 1.87 (1H, brd, J = 13.2 Hz), 1.96 (1H, brd, J = 13.6 Hz), 2.44−2.57 (1H, m), 2.83 (1H, brs), 2.95 (3H, s), 3.40 (1H, brs), 3.53−3.62 (5H, m), 3.73 (1H, brt, J = 9.7 Hz), 3.84 (1H, brs), 4.47 (1H, brs), 7.15 (1H, brt, J = 7.2 Hz), 7.18 (1H, brs), 7.19 (2H, brd, J = 8.1 Hz), 7.27 (2H, brt, J = 7.4 Hz). 13C NMR (151 MHz, DMSO-d6, the minor rotamer’s signals are marked with an asterisk) δ24.05, 24.39*, 26.00, 26.17*, 27.60*, 27.79, 28.68, 30.15*, 37.54, 38.13*, 39.91, 42.99, 51.01, 52.07, 53.90*, 54.49, 61.48, 61.89*, 71.68, 125.68, 126.51, 128.14, 147.34, 155.27*, 156.08. MS (ESI/APCI) mass calculated for [M + H]+ (C21H33N2O5S) requires m/z 424.6, found m/z 425.2. mp 113 °C. Anal. Calcd for C21H32N2O5S: C, 59.41; H, 7.60; N, 6.60. Found: C, 59.45; H, 7.59; N, 6.55. [α] 20 D +16.3 (c 0.1, CHCl3
//////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
 |
|
| Clinical data | |
|---|---|
| Other names | TAK-925 |
| Routes of administration |
Intravenous[1][2] |
| Drug class | Orexin receptor agonist |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H32N2O5S |
| Molar mass | 424.56 g·mol−1 |
| 3D model (JSmol) | |
References
- ^ Jump up to:a b c d e f Jacobson LH, Hoyer D, de Lecea L (January 2022). “Hypocretins (orexins): The ultimate translational neuropeptides”. J Intern Med. doi:10.1111/joim.13406. PMID 35043499.
- ^ Jump up to:a b c “Danavorexton – Takeda”. Adis Insight. Springer Nature Switzerland AG. Retrieved 7 March 2021.
- ^ Evans, R., Hazel, J., Faessel, H., Wu, J., Hang, Y., Alexander, R., … & Hartman, D. (2019). Results of a phase 1, 4-period crossover, placebo-controlled, randomized, single dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of TAK-925, a novel orexin 2 receptor agonist, in sleep-deprived healthy adults, utilizing modafinil as an active comparator. Sleep Medicine, 64, S106. https://scholar.google.com/scholar?cluster=10933819770107034612
- ^ Evans R, Tanaka S, Tanaka S, Touno S, Shimizu K, Sakui S, et al. (December 2019). “A Phase 1 single ascending dose study of a novel orexin 2 receptor agonist, TAK-925, in healthy volunteers (HV) and subjects with narcolepsy type 1 (NT1) to assess safety, tolerability, pharmacokinetics, and pharmacodynamic outcomes”. Sleep Medicine. 64: S105–S106. doi:10.1016/j.sleep.2019.11.290.
- ^ Tong A (6 October 2021). “Takeda flashes red light on ‘breakthrough’ narcolepsy drug after PhII trials turned up mysterious safety signal”. Endpoints News.
External links
///////////////Danavorexton, TAK 925, ORPHAN DRUG, PHASE 1